Assessment and treatment of the withdrawal syndrome in paediatric intensive care units: Systematic review
- PMID: 32000360
- PMCID: PMC7004796
- DOI: 10.1097/MD.0000000000018502
Assessment and treatment of the withdrawal syndrome in paediatric intensive care units: Systematic review
Abstract
Background: Sedoanalgesia secondary iatrogenic withdrawal syndrome (IWS) in paediatric intensive units is frequent and its assessment is complex. Therapies are heterogeneous, and there is currently no gold standard method for diagnosis. In addition, the assessment scales validated in children are scarce. This paper aims to identify and describe both the paediatric diagnostic and assessment tools for the IWS and the treatments for the IWS in critically ill paediatric patients.
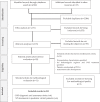
Methods: A systematic review was conducted according to the PRISMA guidelines. This review included descriptive and observational studies published since 2000 that analyzed paediatric scales for the evaluation of the iatrogenic withdrawal syndrome and its treatments. The eligibility criteria included neonates, newborns, infants, pre-schoolers, and adolescents, up to age 18, who were admitted to the paediatric intensive care units with continuous infusion of hypnotics and/or opioid analgesics, and who presented signs or symptoms of deprivation related to withdrawal and prolonged infusion of sedoanalgesia.
Results: Three assessment scales were identified: Withdrawal Assessment Tool-1, Sophia Observation Withdrawal Symptoms, and Opioid and Benzodiazepine Withdrawal Score. Dexmedetomidine, methadone and clonidine were revealed as options for the treatment and prevention of the iatrogenic withdrawal syndrome. Finally, the use of phenobarbital suppressed symptoms of deprivation that are resistant to other drugs.
Conclusions: The reviewed scales facilitate the assessment of the iatrogenic withdrawal syndrome and have a high diagnostic quality. However, its clinical use is very rare. The treatments identified in this review prevent and effectively treat this syndrome. The use of validated iatrogenic withdrawal syndrome assessment scales in paediatrics clinical practice facilitates assessment, have a high diagnostic quality, and should be encouraged, also ensuring nurses' training in their usage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Burastero M, Telechea H, González S, et al. Incidencia del síndrome de abstinencia en niños críticamente enfermos. Arch Pediatr Urug 2017;88:6–11.
-
- Mencía S, Botrán M, López-Herce J, et al. Grupo de Estudio de Sedoanalgesia de la SECIP. Manejo de la sedoanalgesia y de los relajantes musculares en las unidades de cuidados intensivos pediátricos españolas. An Pediatr 2011;74:396–404. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources