Herbal medicine on cancer-related fatigue of lung cancer survivors: Protocol for a systematic review
- PMID: 32000424
- PMCID: PMC7004586
- DOI: 10.1097/MD.0000000000018968
Herbal medicine on cancer-related fatigue of lung cancer survivors: Protocol for a systematic review
Abstract
Background: Lung cancer is one of the most common cancers worldwide, and approximately half of the patients with lung cancer receiving chemotherapy suffer from cancer-related fatigue (CRF). Herbal medicines (HMs) have been used in Oriental countries for centuries as tonics. Various beneficial effects of HM on fatigue and cancer have been reported. However, the effectiveness and safety of HM for CRF in lung cancer patients have not been synthesized. The purpose of this systematic review is to evaluate the effectiveness and safety of HM for CRF in patients with lung cancer, regardless of their cancer type or stage.
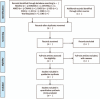
Methods and analysis: A comprehensive search will be conducted in 12 electronic medical databases including 5 English-language databases (Medline via PubMed, EMBASE via Elsevier, the Cochrane Central Register of Controlled Trials [CENTRAL], the Allied and Complementary Medicine Database [AMED] via EBSCO, and the Cumulative Index to Nursing and Allied Health Literature [CINAHL] via EBSCO), 4 Korean-language databases (Oriental Medicine Advanced Searching Integrated System [OASIS], Koreanstudies Information Service System [KISS], Research Information Service System [RISS], and Korea Citation Index [KCI]), 2 Chinese-language databases (China National Knowledge Infrastructure [CNKI] and Wanfang Data), and 1 Japanese-language database (CiNii). Only randomized controlled trials (RCTs) and quasi-RCTs on HM for CRF will be allowed. The severity of fatigue assessed using a validated tool will be considered as theprimary outcome. The secondary outcomes will include the patients' quality of life, activities of daily life, incidence of adverse events, and total effective rate. Two independent researchers will perform the study selection, data extraction, and quality assessment. RevMan version 5.3 will be used for data synthesis. The methodological quality of the included RCTs will be assessed using the Cochrane Collaboration's risk of bias tool. In the meta-analysis, for dichotomous data and continuous data, risk ratio and mean difference, respectively, will be estimated with their 95% confidence intervals. According to the heterogeneity, either a fixed-effects or a random-effects model will be used.
Ethics and dissemination: Ethical approval is not required because individual patient data are not included. The findings of this systematic review will be disseminated through a peer-reviewed publication or conference presentation.
Prospero registration number: CRD42019141660.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Herbal medicine Yukgunja-tang for functional dyspepsia protocol for a systematic review of randomized controlled trials.Medicine (Baltimore). 2018 Oct;97(40):e12555. doi: 10.1097/MD.0000000000012555. Medicine (Baltimore). 2018. PMID: 30290614 Free PMC article.
-
Acupotomy for the treatment of lumbar spinal stenosis: A protocol for a systematic review and meta-analysis.Medicine (Baltimore). 2019 Jan;98(3):e14160. doi: 10.1097/MD.0000000000014160. Medicine (Baltimore). 2019. PMID: 30653158 Free PMC article.
-
Effectiveness and safety of herbal medicine for cancer-related fatigue in lung cancer survivors: A systematic review and meta-analysis.Phytother Res. 2021 Feb;35(2):751-770. doi: 10.1002/ptr.6860. Epub 2020 Sep 14. Phytother Res. 2021. PMID: 32929824
-
Herbal medicine for acute management and rehabilitation of traumatic brain injury: A protocol for a systematic review.Medicine (Baltimore). 2019 Jan;98(3):e14145. doi: 10.1097/MD.0000000000014145. Medicine (Baltimore). 2019. PMID: 30653148 Free PMC article.
-
East asian herbal medicine for the treatment of children with attention deficit hyperactivity disorder: A Systematic Review and Meta-analysis.Explore (NY). 2023 May-Jun;19(3):330-355. doi: 10.1016/j.explore.2022.11.002. Epub 2022 Nov 24. Explore (NY). 2023. PMID: 36463095
Cited by
-
Advances in Stigmasterol on its anti-tumor effect and mechanism of action.Front Oncol. 2022 Dec 12;12:1101289. doi: 10.3389/fonc.2022.1101289. eCollection 2022. Front Oncol. 2022. PMID: 36578938 Free PMC article. Review.
-
Determination of Medicago orbicularis Antioxidant, Antihemolytic, and Anti-Cancerous Activities and Its Augmentation of Cisplatin-Induced Cytotoxicity in A549 Lung Cancer Cells.Plants (Basel). 2024 Feb 2;13(3):442. doi: 10.3390/plants13030442. Plants (Basel). 2024. PMID: 38337975 Free PMC article.
-
Effectiveness and Safety of Chinese Herbal Injections Combined with Fluoropyrimidine and Oxaliplatin-based Chemotherapy for Advanced Colorectal Cancer: A Systematic Review and Meta-analysis of 63 Randomized Controlled Trials.J Cancer. 2021 Oct 25;12(23):7237-7254. doi: 10.7150/jca.60895. eCollection 2021. J Cancer. 2021. PMID: 34729124 Free PMC article.
-
Efficacy of Chinese herbal injections combined with fluoropyrimidine and oxaliplatin-based chemotherapy for advanced colorectal cancer: A protocol for systematic review and meta-analysis of randomized controlled trials.Medicine (Baltimore). 2020 Dec 24;99(52):e23550. doi: 10.1097/MD.0000000000023550. Medicine (Baltimore). 2020. PMID: 33350733 Free PMC article.
-
Progress in the treatment of lung adenocarcinoma by integrated traditional Chinese and Western medicine.Front Med (Lausanne). 2024 Jan 8;10:1323344. doi: 10.3389/fmed.2023.1323344. eCollection 2023. Front Med (Lausanne). 2024. PMID: 38259856 Free PMC article. Review.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. - PubMed
-
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet (London, England) 2017;389:299–311. - PubMed
-
- Chen HL, Liu K, You QS. Self-efficacy, cancer-related fatigue, and quality of life in patients with resected lung cancer. Eur J Cancer Care 2018;27:e12934. - PubMed
-
- Molassiotis A, Lowe M, Blackhall F, et al. A qualitative exploration of a respiratory distress symptom cluster in lung cancer: cough, breathlessness and fatigue. Lung Cancer (Amsterdam, Netherlands) 2011;71:94–102. - PubMed
-
- Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist 2007;12: suppl 1: 4–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

