Clinical and cost effectiveness of a parent mediated intervention to reduce challenging behaviour in pre-schoolers with moderate to severe intellectual disability (EPICC-ID) study protocol: a multi-centre, parallel-group randomised controlled trial
- PMID: 32000729
- PMCID: PMC6993328
- DOI: 10.1186/s12888-020-2451-6
Clinical and cost effectiveness of a parent mediated intervention to reduce challenging behaviour in pre-schoolers with moderate to severe intellectual disability (EPICC-ID) study protocol: a multi-centre, parallel-group randomised controlled trial
Abstract
Background: Children with intellectual disabilities are likely to present with challenging behaviour. Parent mediated interventions have shown utility in influencing child behaviour, although there is a paucity of UK research into challenging behaviour interventions in this population. NICE guidelines favour Stepping Stones Triple P (SSTP) as a challenging behaviour intervention and this trial aims to evaluate its clinical and cost effectiveness in preschool children with moderate to severe intellectual disabilities.
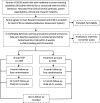
Methods: This trial launched in 2017 at four sites across England, with the aim of recruiting 258 participants (aged 30-59 months). The Intervention Group receive nine weeks of SSTP parenting therapy (six group sessions and three individualised face to face or telephone sessions) in addition to Treatment as Usual, whilst the Treatment as Usual only group receive other available services in each location. Both study groups undergo the study measurements at baseline and at four and twelve months. Outcome measures include parent reports and structured observations of behaviour. Service use and health related quality of life data will also be collected to carry out a cost effectiveness and utility evaluation.
Discussion: Findings from this study will inform policy regarding interventions for challenging behaviour in young children with moderate to severe intellectual disabilities.
Trial registration number: Clinicaltrials.gov, NCT03086876. Registered 22nd March 2017, https://clinicaltrials.gov/ct2/show/NCT03086876.
Keywords: Challenging behaviour; Intellectual disabilities; Parenting interventions; Randomised control trial; SSTP; Stepping stones triple P.
Conflict of interest statement
The authors declare they have no competing interests.
Figures
References
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA; 2013.
-
- Emerson E, Hastings RP, McGill P, Pinney A, Shurlock J. Estimating the number of children in England with learning disabilities and whose behaviours challenge; 2014.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical