Biodegradable polymer nanocomposites for ligament/tendon tissue engineering
- PMID: 32000800
- PMCID: PMC6993465
- DOI: 10.1186/s12951-019-0556-1
Biodegradable polymer nanocomposites for ligament/tendon tissue engineering
Abstract
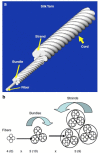
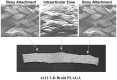
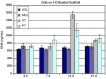
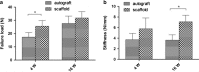
Ligaments and tendons are fibrous tissues with poor vascularity and limited regeneration capacity. Currently, a ligament/tendon injury often require a surgical procedure using auto- or allografts that present some limitations. These inadequacies combined with the significant economic and health impact have prompted the development of tissue engineering approaches. Several natural and synthetic biodegradable polymers as well as composites, blends and hybrids based on such materials have been used to produce tendon and ligament scaffolds. Given the complex structure of native tissues, the production of fiber-based scaffolds has been the preferred option for tendon/ligament tissue engineering. Electrospinning and several textile methods such as twisting, braiding and knitting have been used to produce these scaffolds. This review focuses on the developments achieved in the preparation of tendon/ligament scaffolds based on different biodegradable polymers. Several examples are overviewed and their processing methodologies, as well as their biological and mechanical performances, are discussed.
Keywords: Biodegradability; Nanocomposites; Tendon/ligament tissue engineering.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
















References
Publication types
MeSH terms
Substances
Grants and funding
- TSSIPRO - NORTE-01-0145-FEDER-000015/Programa Operacional Regional do Norte
- UID/CTM/50025/2019/Fundação para a Ciência e a Tecnologia
- UID/CTM/00264/2019/Fundação para a Ciência e a Tecnologia
- SFRH/BD/138244/2018/Fundação para a Ciência e a Tecnologia
- Pest-C/CTM/LA0025/2013 (LA 25 - 2015 - 2018)/Fundação para a Ciência e a Tecnologia
LinkOut - more resources
Full Text Sources

