Neural Correlates of Reinforcement Learning in Mid-lateral Cerebellum
- PMID: 32001108
- PMCID: PMC8015782
- DOI: 10.1016/j.neuron.2019.12.032
Neural Correlates of Reinforcement Learning in Mid-lateral Cerebellum
Erratum in
-
Neural Correlates of Reinforcement Learning in Mid-lateral Cerebellum.Neuron. 2020 Jun 17;106(6):1055. doi: 10.1016/j.neuron.2020.05.021. Neuron. 2020. PMID: 32553199 No abstract available.
Abstract
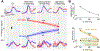
The role of the cerebellum in non-motor learning is poorly understood. Here, we investigated the activity of Purkinje cells (P-cells) in the mid-lateral cerebellum as the monkey learned to associate one arbitrary symbol with the movement of the left hand and another with the movement of the right hand. During learning, but not when the monkey had learned the association, the simple spike responses of P-cells reported the outcome of the animal's most recent decision without concomitant changes in other sensorimotor parameters such as hand movement, licking, or eye movement. At the population level, P-cells collectively maintained a memory of the most recent decision throughout the entire trial. As the monkeys learned the association, the magnitude of this reward-related error signal approached zero. Our results provide a major departure from the current understanding of cerebellar processing and have critical implications for cerebellum's role in cognitive control.
Keywords: Purkinje cell; cerebellum; cognition; complex spike; crus I; crus II; learning; reinforcement learning; simple spike; visuomotor association learning.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



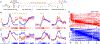

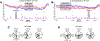
Comment in
-
Research on the cerebellum yields rewards.Nature. 2020 Mar;579(7798):202-203. doi: 10.1038/d41586-020-00636-x. Nature. 2020. PMID: 32152606 No abstract available.
References
-
- ALBUS JS 1971. A theory of cerebellar function. Mathematical Biosciences, 10, 25–61.
-
- BECKER EB & STOODLEY CJ 2013. Autism spectrum disorder and the cerebellum. International review of neurobiology. Elsevier. - PubMed
-
- BUCKNER RL 2013. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron, 80, 807–815. - PubMed
-
- CALIGIORE D, PEZZULO G, BALDASSARRE G, BOSTAN AC, STRICK PL, DOYA K, HELMICH RC, DIRKX M, HOUK J, JÖRNTELL H, LAGO-RODRIGUEZ A, GALEA JM, MIALL RC, POPA T, KISHORE A, VERSCHURE PF, ZUCCA R & HERREROS I 2017. Consensus Paper: Towards a Systems-Level View of Cerebellar Function: the Interplay Between Cerebellum, Basal Ganglia, and Cortex. Cerebellum (London, England), 16, 203–229. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

