Impaired Death Receptor Signaling in Leukemia Causes Antigen-Independent Resistance by Inducing CAR T-cell Dysfunction
- PMID: 32001516
- PMCID: PMC7416790
- DOI: 10.1158/2159-8290.CD-19-0813
Impaired Death Receptor Signaling in Leukemia Causes Antigen-Independent Resistance by Inducing CAR T-cell Dysfunction
Abstract
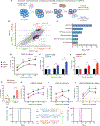
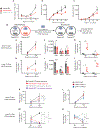
Primary resistance to CD19-directed chimeric antigen receptor T-cell therapy (CART19) occurs in 10% to 20% of patients with acute lymphoblastic leukemia (ALL); however, the mechanisms of this resistance remain elusive. Using a genome-wide loss-of-function screen, we identified that impaired death receptor signaling in ALL led to rapidly progressive disease despite CART19 treatment. This was mediated by an inherent resistance to T-cell cytotoxicity that permitted antigen persistence and was subsequently magnified by the induction of CAR T-cell functional impairment. These findings were validated using samples from two CAR T-cell clinical trials in ALL, where we found that reduced expression of death receptor genes was associated with worse overall survival and reduced T-cell fitness. Our findings suggest that inherent dysregulation of death receptor signaling in ALL directly leads to CAR T-cell failure by impairing T-cell cytotoxicity and promoting progressive CAR T-cell dysfunction. SIGNIFICANCE: Resistance to CART19 is a significant barrier to efficacy in the treatment of B-cell malignancies. This work demonstrates that impaired death receptor signaling in tumor cells causes failed CART19 cytotoxicity and drives CART19 dysfunction, identifying a novel mechanism of antigen-independent resistance to CAR therapy.See related commentary by Green and Neelapu, p. 492.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures




Comment in
-
Not So FASt: Tumor Cells Resisting Death Drive CAR T-cell Dysfunction.Cancer Discov. 2020 Apr;10(4):492-494. doi: 10.1158/2159-8290.CD-20-0037. Cancer Discov. 2020. PMID: 32238396
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

