Cerebrospinal fluid influx drives acute ischemic tissue swelling
- PMID: 32001524
- PMCID: PMC7375109
- DOI: 10.1126/science.aax7171
Cerebrospinal fluid influx drives acute ischemic tissue swelling
Abstract
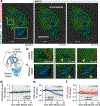
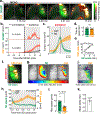
Stroke affects millions each year. Poststroke brain edema predicts the severity of eventual stroke damage, yet our concept of how edema develops is incomplete and treatment options remain limited. In early stages, fluid accumulation occurs owing to a net gain of ions, widely thought to enter from the vascular compartment. Here, we used magnetic resonance imaging, radiolabeled tracers, and multiphoton imaging in rodents to show instead that cerebrospinal fluid surrounding the brain enters the tissue within minutes of an ischemic insult along perivascular flow channels. This process was initiated by ischemic spreading depolarizations along with subsequent vasoconstriction, which in turn enlarged the perivascular spaces and doubled glymphatic inflow speeds. Thus, our understanding of poststroke edema needs to be revised, and these findings could provide a conceptual basis for development of alternative treatment strategies.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures




Comment in
-
Flood of cerebrospinal fluid causes oedema after stroke.Nat Rev Neurol. 2020 Apr;16(4):184-185. doi: 10.1038/s41582-020-0328-4. Nat Rev Neurol. 2020. PMID: 32055022 No abstract available.
-
Opening the floodgates to the brain.Science. 2020 Mar 13;367(6483):1195-1196. doi: 10.1126/science.aba8801. Science. 2020. PMID: 32165571 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

