Neoadjuvant checkpoint blockade for cancer immunotherapy
- PMID: 32001626
- PMCID: PMC7789854
- DOI: 10.1126/science.aax0182
Neoadjuvant checkpoint blockade for cancer immunotherapy
Abstract
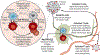
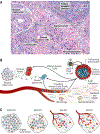
Cancer immunotherapies that target the programmed cell death 1 (PD-1):programmed death-ligand 1 (PD-L1) immune checkpoint pathway have ushered in the modern oncology era. Drugs that block PD-1 or PD-L1 facilitate endogenous antitumor immunity and, because of their broad activity spectrum, have been regarded as a common denominator for cancer therapy. Nevertheless, many advanced tumors demonstrate de novo or acquired treatment resistance, and ongoing research efforts are focused on improving patient outcomes. Using anti-PD-1 or anti-PD-L1 treatment against earlier stages of cancer is hypothesized to be one such solution. This Review focuses on the development of neoadjuvant (presurgical) immunotherapy in the era of PD-1 pathway blockade, highlighting particular considerations for biological mechanisms, clinical trial design, and pathologic response assessments. Findings from neoadjuvant immunotherapy studies may reveal pathways, mechanisms, and molecules that can be cotargeted in new treatment combinations to increase anti-PD-1 and anti-PD-L1 efficacy.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

