Therapeutic targeting of preleukemia cells in a mouse model of NPM1 mutant acute myeloid leukemia
- PMID: 32001657
- PMCID: PMC7754791
- DOI: 10.1126/science.aax5863
Therapeutic targeting of preleukemia cells in a mouse model of NPM1 mutant acute myeloid leukemia
Abstract
The initiating mutations that contribute to cancer development are sometimes present in premalignant cells. Whether therapies targeting these mutations can eradicate premalignant cells is unclear. Acute myeloid leukemia (AML) is an attractive system for investigating the effect of preventative treatment because this disease is often preceded by a premalignant state (clonal hematopoiesis or myelodysplastic syndrome). In Npm1c/Dnmt3a mutant knock-in mice, a model of AML development, leukemia is preceded by a period of extended myeloid progenitor cell proliferation and self-renewal. We found that this self-renewal can be reversed by oral administration of a small molecule (VTP-50469) that targets the MLL1-Menin chromatin complex. These preclinical results support the hypothesis that individuals at high risk of developing AML might benefit from targeted epigenetic therapy in a preventative setting.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures


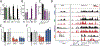
Comment in
-
Preleukemic Cells Can Be Eliminated in NPM1-Mutant Acute Myeloid Leukemia Models.Cancer Discov. 2020 Mar;10(3):341. doi: 10.1158/2159-8290.CD-RW2020-021. Epub 2020 Feb 7. Cancer Discov. 2020. PMID: 32033969
References
-
- Falini B. et al. Cytoplasmic Nucleophosmin in Acute Myelogenous Leukemia with a Normal Karyotype. N. Engl. J. Med 352, 254–266 (2005). - PubMed
-
- Schlenk RF et al. Mutations and Treatment Outcome in Cytogenetically Normal Acute Myeloid Leukemia. N. Engl. J. Med 358, 1909–1918 (2008). - PubMed
-
- Döhner H, Weisdorf DJ & Bloomfield CD Acute Myeloid Leukemia. N. Engl. J. Med 373, 1136–1152 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

