Cerebrospinal fluid lipocalin 2 as a novel biomarker for the differential diagnosis of vascular dementia
- PMID: 32001681
- PMCID: PMC6992814
- DOI: 10.1038/s41467-020-14373-2
Cerebrospinal fluid lipocalin 2 as a novel biomarker for the differential diagnosis of vascular dementia
Abstract
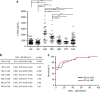
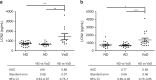
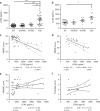
The clinical diagnosis of vascular dementia (VaD) is based on imaging criteria, and specific biochemical markers are not available. Here, we investigated the potential of cerebrospinal fluid (CSF) lipocalin 2 (LCN2), a secreted glycoprotein that has been suggested as mediating neuronal damage in vascular brain injuries. The study included four independent cohorts with a total n = 472 samples. LCN2 was significantly elevated in VaD compared to controls, Alzheimer's disease (AD), other neurodegenerative dementias, and cognitively unimpaired patients with cerebrovascular disease. LCN2 discriminated VaD from AD without coexisting VaD with high accuracy. The main findings were consistent over all cohorts. Neuropathology disclosed a high percentage of macrophages linked to subacute infarcts, reactive astrocytes, and damaged blood vessels in multi-infarct dementia when compared to AD. We conclude that CSF LCN2 is a promising candidate biochemical marker in the differential diagnosis of VaD and neurodegenerative dementias.
Conflict of interest statement
K.B. has served as a consultant or on the advisory boards for Alzheon, BioArctic, Biogen, Eli Lilly, Fujirebio Europe, IBL International, Merck, Novartis, Pfizer, and Roche Diagnostics, all un-related to the data presented in the present paper. O.H. has acquired research support (for the institution) from Roche, GE Healthcare, Biogen, AVID Radiopharmaceuticals, Fujirebio, and Euroimmun. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Biogen, Roche, and Fujirebio. H.Z. has served on scientific advisory boards for Eli Lilly, Roche Diagnostics, CogRx, Samumed, and Wave, has received travel support from Teva and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. C.P. is member of the International Advisory Boards of Lilly, is consultant of Fujiribio, ALZOHIS, NEUROIMMUNE, and GILEAD and is involved as investigator in several clinical trials for Roche, Esai, Lilly, Biogen, Astra-Zeneca, Lundbeck, and Neuroimmune. J.D. is an investigator in several passive anti-amyloid immunotherapies and other clinical trials for Roche, Eisai, Lilly, Biogen, Astra-Zeneca, Lundbeck. The remaining authors report no biomedical financial interests or potential conflicts of interest.
Figures