Carbon dioxide electroreduction on single-atom nickel decorated carbon membranes with industry compatible current densities
- PMID: 32001699
- PMCID: PMC6992760
- DOI: 10.1038/s41467-020-14402-0
Carbon dioxide electroreduction on single-atom nickel decorated carbon membranes with industry compatible current densities
Abstract
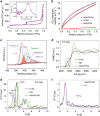
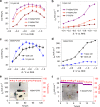
Carbon dioxide electroreduction provides a useful source of carbon monoxide, but comparatively few catalysts could be sustained at current densities of industry level. Herein, we construct a high-yield, flexible and self-supported single-atom nickel-decorated porous carbon membrane catalyst. This membrane possesses interconnected nanofibers and hierarchical pores, affording abundant effective nickel single atoms that participate in carbon dioxide reduction. Moreover, the excellent mechanical strength and well-distributed nickel atoms of this membrane combines gas-diffusion and catalyst layers into one architecture. This integrated membrane could be directly used as a gas diffusion electrode to establish an extremely stable three-phase interface for high-performance carbon dioxide electroreduction, producing carbon monoxide with a 308.4 mA cm-2 partial current density and 88% Faradaic efficiency for up to 120 h. We hope this work will provide guidance for the design and application of carbon dioxide electro-catalysts at the potential industrial scale.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Wang J, et al. A Water-soluble Cu complex as molecular catalyst for electrocatalytic CO2 reduction on graphene-based electrodes. Adv. Energy Mater. 2019;9:1803151. doi: 10.1002/aenm.201803151. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

