Use of biomaterials for sustained delivery of anti-VEGF to treat retinal diseases
- PMID: 32001821
- PMCID: PMC7376230
- DOI: 10.1038/s41433-020-0770-y
Use of biomaterials for sustained delivery of anti-VEGF to treat retinal diseases
Erratum in
-
Correction: Use of biomaterials for sustained delivery for anti-VEGF to treat retinal diseases.Eye (Lond). 2020 Aug;34(8):1486. doi: 10.1038/s41433-020-0980-3. Eye (Lond). 2020. PMID: 32444861 Free PMC article.
Abstract
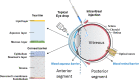
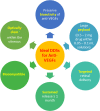
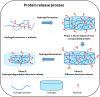
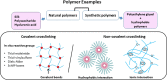
Anti-vascular endothelial growth factors (anti-VEGF) have become the most common treatment modality for many retinal diseases. These include neovascular age-related macular degeneration (n-AMD), proliferative diabetic retinopathy (PDR) and retinal vein occlusions (RVO). However, these drugs are administered via intravitreal injections that are associated with sight-threatening complications. The most feared of these complications is endophthalmitis, a severe infection of the eye with extremely poor visual outcomes. Patients with retinal diseases typically have to undergo multiple injections before achieving the desired therapeutic effect. Each injection incurs the risk of the sight-threatening complications. As such, there has been great interest in developing sustained delivery platforms for anti-VEGF agents to the posterior segment of the eye. In recent years, there have been various strategies that have been conceptualised. These include non-biodegradable implants, nano-formulations and hydrogels. In this review, the barriers of drug delivery to the posterior segment of the eye will be explained. The characteristics of an ideal sustained delivery platform will then be discussed. Finally, the current available strategies will be analysed with the above-mentioned characteristics in mind to determine the advantages and disadvantages of each sustained drug delivery modality. Through the above, this review attempts to provide an overview of the sustained delivery platforms in their various phases of development.
摘要: 抗血管内皮生长因子 (Anti-vascular endothelial growth factors, anti-VEGF) 已成为治疗视网膜疾病最常见的治疗方法, 其中包括新生血管性年龄相关性黄斑变性 (neovascular age-related macular degeneration, n-AMD) 、增殖型糖尿病视网膜病 (proliferative diabetic retinopathy, PDR) 和视网膜静脉阻塞 (retinal vein occlusions, RVO) 。然而, 给药途径为玻璃体腔内注射, 因此可能会发生威胁视力的并发症。其中最可怕的并发症是眼内炎。眼内炎为严重的眼部感染, 视力预后极差。罹患视网膜疾病的患者通常需要多次眼内注射达到预期的治疗效果。每次注射都会有威胁视力并发症发生的风险。因此, 人们对抗VEGF药物应用于眼后节的持续性给药系统的研发非常关注。近年来, 新的治疗策略新的治疗理念不断涌现, 其中包括非生物降解性植入物、纳米制剂和水凝胶。本文我们将阐述药物输送至后节的屏障, 随后探讨理想的持续性输送给药系统的特点, 最后将结合上述特点分析现有的策略, 以确定每种持续性药物输送方式的优缺点。本文旨在综述持续性输送给药系统的各个发展阶段的概况。.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Comment in
-
Response to 'Comment on: "Use of biomaterials for sustained delivery of anti-VEGF to treat retinal diseases"'.Eye (Lond). 2021 Mar;35(3):1026-1027. doi: 10.1038/s41433-020-0983-0. Epub 2020 May 28. Eye (Lond). 2021. PMID: 32461567 Free PMC article. No abstract available.
-
Comment on "Use of biomaterials for sustained delivery of anti-VEGF to treat retinal diseases".Eye (Lond). 2021 Mar;35(3):1024-1025. doi: 10.1038/s41433-020-0982-1. Epub 2020 May 28. Eye (Lond). 2021. PMID: 32467630 Free PMC article. No abstract available.
References
-
- Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112:1035–47. - PubMed
-
- Mason JO, 3rd, White MF, Feist RM, Thomley ML, Albert MA, Persaud TO, et al. Incidence of acute onset endophthalmitis following intravitreal bevacizumab (Avastin) injection. Retina. 2008;28:564–7. - PubMed
-
- Bhavsar AR, Googe JM, Jr, Stockdale CR, Bressler NM, Brucker AJ, Elman MJ, et al. Risk of endophthalmitis after intravitreal drug injection when topical antibiotics are not required: the diabetic retinopathy clinical research network laser-ranibizumab-triamcinolone clinical trials. Arch Ophthalmol. 2009;127:1581–3. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

