Toxoplasma gondii GRA8-derived peptide immunotherapy improves tumor targeting of colorectal cancer
- PMID: 32002124
- PMCID: PMC6967779
- DOI: 10.18632/oncotarget.27417
Toxoplasma gondii GRA8-derived peptide immunotherapy improves tumor targeting of colorectal cancer
Abstract
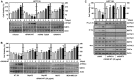
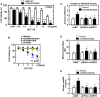
Targeted tumor and efficient, specific biological drug delivery in vivo has been one of the main challenges in protein-based cancer-targeted therapies. Mitochondria are potential therapeutic targets for various anti-cancer drugs. We have previously reported that protein kinase Cα-mediated phosphorylation of Toxoplasma gondii GRA8 is required for mitochondrial trafficking and regulating the interaction of the C-terminal of GRA8 with ATP5A1/SIRT3 in mitochondria. Furthermore, SIRT3 facilitates ATP5A1 deacetylation, mitochondrial activation, and subsequent antiseptic activity in vivo. Herein we developed a recombinant acidity-triggered rational membrane (ATRAM)-conjugated multifunctional GRA8 peptide (rATRAM-G8-M/AS) comprising ATRAM as the cancer-targeting cell-penetrating peptide, and essential/minimal residues for mitochondrial targeting or ATP5A1/SIRT3 binding. This peptide construct showed considerably improved potency about cancer cell death via mitochondria activity and biogenesis compared with rGRA8 alone in HCT116 human carcinoma cells, reaching an IC50 value of up to 200-fold lower in vitro and 500-fold lower in vivo. Notably, rATRAM-G8-M/AS treatment showed significant therapeutic effects in a mouse xenograft model through mitochondrial metabolic resuscitation, and it produced negligible immunogenicity and immune responses in vivo. Thus, these results demonstrate that rATRAM-G8-M/AS represents a useful therapeutic strategy against tumors, particularly colon cancer. This strategy represents an urgently needed paradigm shift for therapeutic intervention.
Keywords: Toxoplasma gondii GRA8 peptide; metabolism; mitochondria; tumor-targeting.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that they have no conflicts of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

