Clinical features of angioedema induced by renin-angiotensin-aldosterone system inhibition: a retrospective analysis of 84 patients
- PMID: 32002148
- PMCID: PMC6968333
- DOI: 10.1080/20009666.2019.1698259
Clinical features of angioedema induced by renin-angiotensin-aldosterone system inhibition: a retrospective analysis of 84 patients
Abstract
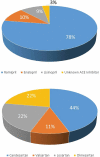
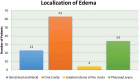
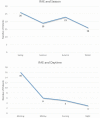
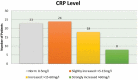
Background and objectives: Bradykinin-mediated angioedema (AE) induced by antihypertensive drugs primarily affect the head and neck region and may occur even after several years of uneventful treatment. Many facts about the clinical course remain unknown. Diagnosis is not easy, as the clinical appearance resembles allergic AE. No specific diagnostic markers are known and no officially approved treatment is currently available. Methods: All patients who presented to the ORL department between 2010 and 2016 with acute AE were included. Those with a history of renin-angiotensin-aldosterone system (RAAS) blocker intake were defined as RAE and their pathophysiological characteristics and clinical course of the disease were analyzed. Results: A total of 84 patients (median age of 71 years) with RAE was identified. The majority (80%) was on ACE inhibition. The oral cavity was most often affected. Nearly 60% were medicated for more than 1 year before AE occurred. RAE occurred more often during the morning hours. The necessity for emergency intubation and/or tracheostomy was nine times higher in patients with acute RAE compared to patients with AE due to other reasons. Conclusions: Event-free, long-term therapy with an RAAS blocker before the first development of edema does not exclude RAE. RAE is associated with an increased risk for emergency airway management. Abbreviations ACE: Angiotensin Converting Enzyme; ACEi AE: ACE inhibitor-induced angioedema; AE: Angioedema; ARB: Angiotensin II receptor 1 blocker; C1 INH: C1 Inhibitor; CI: Confidence Interval; CRP: C-reactive protein; DPP IV: Dipeptidyl peptidase IV; ENT: Ear, Nose and Throat; HAE: Hereditary Angioedema; ICD 10: International Statistical Classification of Diseases and Related Health Problems, 10th Edition; OR: Odds Ratio; ORL: Otorhinolaryngology; RAAS: Renin-Angiotensin-Aldosterone System; RAE: RAAS-blocker-induced angioedema.
Keywords: ACE inhibitor; Angioedema; RAAS; bradykinin.
© 2019 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group on behalf of Greater Baltimore Medical Center.
Figures
References
-
- Wilkin JK, Hammond JJ, Kirkendall WM.. The captopril-induced eruption. A possible mechanism: cutaneous kinin potentiation. Arch Dermatol. 1980;116:902. - PubMed
-
- Bas M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372:418–425. - PubMed
-
- Bas M, Greve J, Strassen U, et al. Angioedema induced by cardiovascular drugs: new players join old friends. Allergy. 2015;70:1196–1200. - PubMed
-
- Mujer MTP, Rai MP, Nemakayala DR, et al. Angioedema of the small bowel caused by lisinopril. Drug Ther Bull. 2019;57:14–15 No commercial re-use. See rights and permissions. Published by BMJ. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
