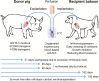
Genetically modified pigs as donors of cells, tissues, and organs for xenotransplantation
- PMID: 32002258
- PMCID: PMC6951927
- DOI: 10.1093/af/vfz014
Genetically modified pigs as donors of cells, tissues, and organs for xenotransplantation
Keywords: heart; organ donor; pancreatic islet; pig; xenotransplantation.
Figures


References
-
- Aron Badin R., Vadori M., Vanhove B., Nerriere-Daguin V., Naveilhan P., Neveu I., Jan C., Lévèque X., Venturi E., Mermillod P., et al.2016. Cell therapy for parkinson’s disease: a translational approach to assess the role of local and systemic immunosuppression. Am. J. Transplant. 16:2016–2029. doi:10.1111/ajt.13704 - DOI - PubMed
-
- Bähr A., Käser T., Kemter E., Gerner W., Kurome M., Baars W., Herbach N., Witter K., Wünsch A., Talker S. C., et al.2016. Ubiquitous LEA29Y expression blocks T cell co-stimulation but permits sexual reproduction in genetically modified pigs. Plos One. 11:e0155676. doi:10.1371/journal.pone.0155676 - DOI - PMC - PubMed
-
- Bartlett S. T., Markmann J. F., Johnson P., Korsgren O., Hering B. J., Scharp D., Kay T. W., Bromberg J., Odorico J. S., Weir G. C., et al.2016. Report from IPITA-TTS opinion leaders meeting on the future of β-cell replacement. Transplantation. 100(Suppl 2):S1–44. doi:10.1097/TP.0000000000001055 - DOI - PMC - PubMed
-
- Buermann A., Petkov S., Petersen B., Hein R., Lucas-Hahn A., Baars W., Brinkmann A., Niemann H., and Schwinzer R.. 2018. Pigs expressing the human inhibitory ligand PD-L1 (CD 274) provide a new source of xenogeneic cells and tissues with low immunogenic properties. Xenotransplantation. 25:e12387. doi:10.1111/xen.12387 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
