Efficacy and safety of anticancer drug combinations: a meta-analysis of randomized trials with a focus on immunotherapeutics and gene-targeted compounds
- PMID: 32002305
- PMCID: PMC6959453
- DOI: 10.1080/2162402X.2019.1710052
Efficacy and safety of anticancer drug combinations: a meta-analysis of randomized trials with a focus on immunotherapeutics and gene-targeted compounds
Abstract
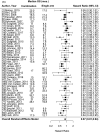
Hundreds of trials are being conducted to evaluate combination of newer targeted drugs as well as immunotherapy. Our aim was to compare efficacy and safety of combination versus single non-cytotoxic anticancer agents. We searched PubMed (01/01/2001 to 03/06/2018) (and, for immunotherapy, ASCO and ESMO abstracts (2016 through March 2018)) for randomized clinical trials that compared a single non-cytotoxic agent (targeted, hormonal, or immunotherapy) versus a combination with another non-cytotoxic partner. Efficacy and safety endpoints were evaluated in a meta-analysis using a linear mixed-effects model (guidelines per PRISMA Report).We included 95 randomized comparisons (single vs. combination non-cytotoxic therapies) (59.4%, phase II; 41.6%, phase III trials) (29,175 patients (solid tumors)). Combinations most frequently included a hormonal agent and a targeted small molecule (23%). Compared to single non-cytotoxic agents, adding another non-cytotoxic drug increased response rate (odds ratio [OR]=1.61, 95%CI 1.40-1.84)and prolonged progression-free survival (hazard ratio [HR]=0.75, 95%CI 0.69-0.81)and overall survival (HR=0.87, 95%CI 0.81-0.94) (all p<0.001), which was most pronounced for the association between immunotherapy combinations and longer survival. Combinations also significantlyincreased the risk of high-grade toxicities (OR=2.42, 95%CI 1.98-2.97) (most notably for immunotherapy and small molecule inhibitors) and mortality at least possibly therapy related (OR: 1.33, 95%CI 1.15-1.53) (both p<0.001) (absolute mortality = 0.90% (single agent) versus 1.31% (combinations)) compared to single agents. In conclusion, combinations of non-cytotoxic drugs versus monotherapy in randomized cancer clinical trials attenuated safety, but increased efficacy, with the balance tilting in favor of combination therapy, based on the prolongation in survival.
Keywords: Combination therapy; hormonal therapy; immunotherapy; solid tumors; targeted therapy.
© 2020 The Author(s). Published with license by Taylor & Francis Group, LLC.
Figures




Similar articles
-
Comparison of Long-term Survival Benefits in Trials of Immune Checkpoint Inhibitor vs Non-Immune Checkpoint Inhibitor Anticancer Agents Using ASCO Value Framework and ESMO Magnitude of Clinical Benefit Scale.JAMA Netw Open. 2019 Jul 3;2(7):e196803. doi: 10.1001/jamanetworkopen.2019.6803. JAMA Netw Open. 2019. PMID: 31290990 Free PMC article.
-
Dosing Three-Drug Combinations That Include Targeted Anti-Cancer Agents: Analysis of 37,763 Patients.Oncologist. 2017 May;22(5):576-584. doi: 10.1634/theoncologist.2016-0357. Epub 2017 Apr 19. Oncologist. 2017. PMID: 28424323 Free PMC article.
-
Doublet Versus Single Agent as Second-Line Treatment for Advanced Gastric Cancer: A Meta-Analysis of 10 Randomized Controlled Trials.Medicine (Baltimore). 2016 Feb;95(8):e2792. doi: 10.1097/MD.0000000000002792. Medicine (Baltimore). 2016. PMID: 26937908 Free PMC article. Review.
-
Efficacy of combining targeted therapy with pemetrexed or docetaxel as second-line treatment in patients with advanced non-small-cell lung cancer: a meta-analysis of 14 randomized controlled trials.Curr Med Res Opin. 2014 Nov;30(11):2295-304. doi: 10.1185/03007995.2014.909392. Epub 2014 Apr 30. Curr Med Res Opin. 2014. PMID: 24701984 Review.
-
Patients with sarcomatoid renal cell carcinoma - re-defining the first-line of treatment: A meta-analysis of randomised clinical trials with immune checkpoint inhibitors.Eur J Cancer. 2020 Sep;136:195-203. doi: 10.1016/j.ejca.2020.06.008. Epub 2020 Jul 23. Eur J Cancer. 2020. PMID: 32712550
Cited by
-
A pan-cancer screen identifies drug combination benefit in cancer cell lines at the individual and population level.Cell Rep Med. 2024 Aug 20;5(8):101687. doi: 10.1016/j.xcrm.2024.101687. Cell Rep Med. 2024. PMID: 39168097 Free PMC article.
-
Novel clinical trial designs emerging from the molecular reclassification of cancer.CA Cancer J Clin. 2025 May-Jun;75(3):243-267. doi: 10.3322/caac.21880. Epub 2025 Jan 22. CA Cancer J Clin. 2025. PMID: 39841128 Free PMC article. Review.
-
Synergistic effects of abietic acid combined with doxorubicin on apoptosis induction in a human colorectal cancer cell line.Sci Rep. 2025 May 8;15(1):16102. doi: 10.1038/s41598-025-99616-2. Sci Rep. 2025. PMID: 40341222 Free PMC article.
-
Challenges of applying multicellular tumor spheroids in preclinical phase.Cancer Cell Int. 2021 Mar 4;21(1):152. doi: 10.1186/s12935-021-01853-8. Cancer Cell Int. 2021. PMID: 33663530 Free PMC article. Review.
-
Open peer review commentary on building clinically relevant outcomes across the Alzheimer's disease spectrum.Alzheimers Dement (N Y). 2021 Jun 26;7(1):e12192. doi: 10.1002/trc2.12192. eCollection 2021. Alzheimers Dement (N Y). 2021. PMID: 34195351 Free PMC article. No abstract available.
