The novel Chinese coronavirus (2019-nCoV) infections: Challenges for fighting the storm
- PMID: 32003000
- PMCID: PMC7163647
- DOI: 10.1111/eci.13209
The novel Chinese coronavirus (2019-nCoV) infections: Challenges for fighting the storm
Conflict of interest statement
Outside the submitted work, MB serves on scientific advisory boards for Angelini, AstraZeneca, Bayer, Cubist, Pfizer, Menarini, MSD, Nabriva, Paratek, Roche, Shionogi, Tetraphase, The Medicine Company and Astellas Pharma Inc and has received funding for travel or speaker honoraria from Algorithm, Angelini, Astellas Pharma Inc, AstraZeneca, Cubist, Pfizer, MSD, Gilead Sciences, Menarini, Novartis, Ranbaxy, Teva. Outside the submitted work, DRG reports an unconditional grant from MSD Italia and honoraria from Stepstone Pharma GmbH.
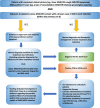
Figures
References
-
- European Centre for Disease Prevention and Control . Geographical distribution of 2019-nCov cases globally. https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases [Accessed 30 Jan 2020; 15:40 CET].
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

