Drug-interactive mPEG- b-PLA-Phe(Boc) micelles enhance the tolerance and anti-tumor efficacy of docetaxel
- PMID: 32003299
- PMCID: PMC7034090
- DOI: 10.1080/10717544.2020.1718245
Drug-interactive mPEG- b-PLA-Phe(Boc) micelles enhance the tolerance and anti-tumor efficacy of docetaxel
Abstract
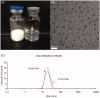
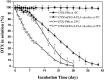
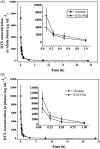
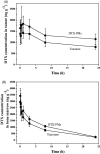
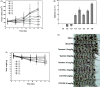
Docetaxel (DTX) is one of the most promising chemotherapeutic agents for a variety of solid tumors. However, the clinical efficacy of the marketed formulation, Taxotere®, is limited due to its poor aqueous solubility, side effects caused by the emulsifier, and low selective DTX distribution in vivo. Here a facile, well-defined, and easy-to-scale up DTX-loaded N-(tert-butoxycarbonyl)-L-phenylalanine end-capped methoxy-poly(ethylene glycol)-block-poly(D,L-lactide) (mPEG-b-PLA-Phe(Boc)) micelles (DTX-PMs) were prepared in an effort to develop a less toxic and more efficacious docetaxel formulation. The physicochemical properties, pharmacokinetics, biodistribution, and in vivo anti-tumor efficacy were evaluated in comparison to the marketed DTX formulation Taxotere®. DTX was successfully encapsulated in the hydrophobic micellar core with a high encapsulation efficiency (> 95%) and a high drug loading capacity (4.81 ± 0.08%). DTX-PMs exhibited outstanding stability in the aqueous environment due to the strong interactions between the terminal amino acid residues and docetaxel. The pharmacokinetic study in Sprague-Dawley rats revealed higher DTX concentrations in both whole blood and plasma for the group treated with DTX-PMs than that treated with Taxotere® due to the improved stability of the micellar formulation. In human non-small cell lung cancer (A549) tumor-bearing Balb/c nude mice, DTX-PMs significantly improved DTX accumulation and stalled DTX elimination in tumors than in bone marrow. Furthermore, only by half of the DTX dosage, our DTX/mPEG-b-PLA-Phe(Boc) micelles can achieve similar therapeutic effects as Taxotere®. Altogether, DTX-PMs hold great promise as a simple and effective drug delivery system for cancer chemotherapy.
Keywords: Polymeric micelles; anti-tumor efficacy; docetaxel.
Figures


References
-
- Balakrishnan P, Song CK, Jahn A, Cho HJ. (2016). Ceramide and N,N,N-trimethylphytosphingosine-iodide (TMP-I)-based lipid nanoparticles for cancer therapy. Pharm Res 33:206–16. - PubMed
-
- Cavallaro G, Craparo EF, Sardo C, et al. (2015). PHEA-PLA biocompatible nanoparticles by technique of solvent evaporation from multiple emulsions. Int J Pharm 495:719–27. - PubMed
-
- Gao Y, Chen L, Gu W, et al. (2008). Targeted nanoassembly loaded with docetaxel improves intracellular drug delivery and efficacy in murine breast cancer model. Mol Pharm 5:1044–54. - PubMed
-
- Gaucher G, Marchessault RH, Leroux JC. (2010). Polyester-based micelles and nanoparticles for the parenteral delivery of taxanes. J Control Release 143:2–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
