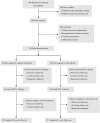
A single-blind randomized control trial of trigonal versus nontrigonal Botulinum toxin-A injections for patients with urinary incontinence and poor bladder compliance secondary to spinal cord injury
- PMID: 32003644
- PMCID: PMC8477943
- DOI: 10.1080/10790268.2020.1712892
A single-blind randomized control trial of trigonal versus nontrigonal Botulinum toxin-A injections for patients with urinary incontinence and poor bladder compliance secondary to spinal cord injury
Abstract
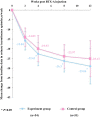
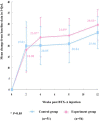
Objective: To evaluate the effect of trigonal Botulinum toxin-A (BTX-A) injections on patients with urinary incontinence (UI) and poor bladder compliance (BC) secondary to spinal cord injury (SCI).Design: A single-blind randomized control trial.Setting: Department of urology in three hospitals.Participants: SCI patients with UI and poor BC were randomly assigned to either the experimental group or the control group.Interventions: The experimental group received an injection of 240 U BTX-A into the detrusor plus 60 U BTX-A into the trigone, while the control group received 300 U BTX-A into the detrusor sparing the trigone.Outcome Measures: Video urodynamic outcomes, including vesicoureteric reflux (VUR), detrusor leak point pressure (DLPP), and detrusor leak point volume (DLPV), were measured at baseline and week 12. UI episodes, voiding volume, and Incontinence Quality of Life (I-QoL) were assessed at baseline, week 2, 4, 8 and 12.Results: No patient reported new-onset VUR. Compared with baseline data, a significant improvement was achieved in both groups, whereas compared with DLPP and DLPV, a significant difference was noted between the two groups 12 weeks after injection. In the experimental group, the improvement of mean weekly UI episodes, voiding volume, and I-QoL were significantly better than those in the control group at 4, 8, and 12 weeks, respectively (all P < 0.05). Systemic complications of BTX-A injection were not reported.Conclusion: Trigonal BTX-A injection is more effective and safer than nontrigonal BTX-A injection for SCI patients with UI secondary to neurogenic-poor BC and does not result in VUR.
Keywords: Bladder trigone; Botulinum toxin-A; Poor bladder compliance; Randomized controlled trial; Spinal cord injury.
Figures



References
-
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. . The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167–78. doi: 10.1002/nau.10052 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
