The Interleukin-33/ST2 axis promotes glioma mesenchymal transition, stemness and TMZ resistance via JNK activation
- PMID: 32003751
- PMCID: PMC7053587
- DOI: 10.18632/aging.102707
The Interleukin-33/ST2 axis promotes glioma mesenchymal transition, stemness and TMZ resistance via JNK activation
Abstract
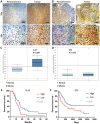
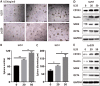
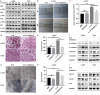
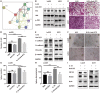
IL-33 is an important member of the IL-1 family which has pleiotropic activities in innate and adaptive immune responses. Recently, some researchers have focused on the function of cellular immunity in the development of tumor. The biological role of IL-33 in glioma is poorly understood. In this study, we showed that glioma cells and tissues expressed higher levels of IL-33 and its receptor ST2 compared to normal brain. Clinically, IL-33 expression was associated with poor survival in patients with glioma. Administration of human IL-33 enhanced cell migration, invasion, epithelial to mesenchymal transition and stemness. Anti-ST2 blocked these effects of IL-33 on tumor. Mechanistically, IL-33 activated JNK signaling pathway via ST2 and increased the expression of key transcription factors that controlled the process of EMT and stemness. Moreover, IL-33 prevented temozolomide induced tumor apoptosis. Anti-ST2 or knockdown IL-33 increased the sensitivity of tumor to temozolomide. Thus, targeting the IL-33/ST2 axis may offer an opportunity to the treatment of glioma patients.
Keywords: EMT; IL-33; glioma; stemness; temozolomide.
Conflict of interest statement
Figures



References
-
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005; 23:479–90. 10.1016/j.immuni.2005.09.015 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

