Antithrombotic effects of heme-degrading and heme-binding proteins
- PMID: 32004074
- PMCID: PMC7099452
- DOI: 10.1152/ajpheart.00280.2019
Antithrombotic effects of heme-degrading and heme-binding proteins
Abstract
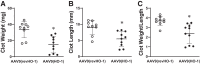
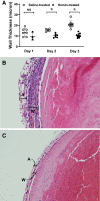
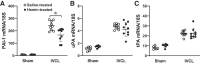
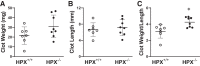
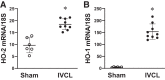
In the murine venous thrombosis model induced by ligation of the inferior vena cava (IVCL), genetic deficiency of heme oxygenase-1 (HO-1) increases clot size. This study examined whether induction of HO-1 or administration of its products reduces thrombosis. Venous HO-1 upregulation by gene delivery reduced clot size, as did products of HO activity, biliverdin, and carbon monoxide. Induction of HO-1 by hemin reduced clot formation, clot size, and upregulation of plasminogen activator inhibitor-1 (PAI-1) that occurs in the IVCL model, while leaving urokinase plasminogen activator (uPA) and tissue plasminogen activator (tPA) expression unaltered. The reductive effect of hemin on clot size required HO activity. The IVCL model exhibited relatively high concentrations of heme that peaked just before maximum clot size, then declined as clot size decreased. Administration of hemin decreased heme concentration in the IVCL model. HO-2 mRNA was induced twofold in the IVCL model (vs. 40-fold HO-1 induction), but clot size was not increased in HO-2-/- mice compared with HO-2+/+ mice. Hemopexin, the major heme-binding protein, was induced in the IVCL model, and clot size was increased in hemopexin-/- mice compared with hemopexin+/+ mice. We conclude that in the IVCL model, the heme-degrading protein HO-1 and HO products inhibit thrombus formation, as does the heme-binding protein, hemopexin. The reductive effects of hemin administration require HO activity and are mediated, in part, by reducing PAI-1 upregulation in the IVCL model. We speculate that HO-1, HO, and hemopexin reduce clot size by restraining the increase in clot concentration of heme (now recognized as a procoagulant) that otherwise occurs.NEW & NOTEWORTHY This study provides conclusive evidence that two proteins, one heme-degrading and the other heme-binding, inhibit clot formation. This may serve as a new therapeutic strategy in preventing and treating venous thromboembolic disease.
Keywords: bile pigments; carbon monoxide; heme oxygenase-1; hemopexin; murine model; venous thromboembolic disease.
Conflict of interest statement
G. M. Vercellotti and J. D. Belcher receive research funding from CSL Behring and Mitobridge/Astellas. Dr. Regan has received research funding from CSL Behring.
Figures



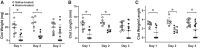





References
-
- Alotaibi GS, Wu C, Senthilselvan A, McMurtry MS. Secular trends in incidence and mortality of acute venous thromboembolism: the AB-VTE population-based study. Am J Med 129: 819–825, 2016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

