Measuring retention in HIV care: the impact of data sources and definitions using routine data
- PMID: 32004202
- PMCID: PMC7109335
- DOI: 10.1097/QAD.0000000000002478
Measuring retention in HIV care: the impact of data sources and definitions using routine data
Abstract
Objectives: Measuring retention is critical for antiretroviral therapy (ART) management and program monitoring; however, many definitions and data sources, usually from single health facilities, are used. We used routine electronic data, linked across facilities, to examine the impact of definitions and data sources on retention estimates among women in Cape Town, South Africa.
Design: Retrospective cohort study.
Methods: We compiled routine electronic laboratory, pharmacy and clinic visit data for 617 women who started ART during pregnancy (2013-2014) and estimated 24-month retention using different definitions and data sources. We used logistic regression to assess consistency of associations between risk factors and retention, and receiver operating characteristics analyses to describe how different retention estimates predict viremia at 12 months on ART.
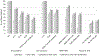
Results: Using all available data sources, retention ranged from 41% (no gap >180 days) to 72% (100% 12-month visit constancy). Laboratory data (expected infrequently) underestimated retention compared with clinic visit data that identified more than 80% of women considered retained in all definitions. In all estimates, associations with known risk factors for nonretention remained consistent and retention declined over time: 77, 65 and 58% retained using all data sources in months 6-12, 12-18 and 18-24, respectively (P < 0.001). The 180-day gap definition was most strongly associated with viremia (odds ratio 24.3 95% confidence interval 12.0-48.9, all data sources).
Conclusion: Researchers must carefully consider the most appropriate retention definition and data source depending on available data. Presenting more than one approach may be warranted to obtain estimates that are context-appropriate and comparable across settings.
Conflict of interest statement
Disclosures
The authors declare no conflicts of interest in this work.
Figures


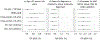
References
-
- UNAIDS. 90-90-90 An ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: UNAIDS; 2014. http://www.unaids.org/en/resources/909090
-
- UNAIDS. Miles to Go - closing gaps, breaking barriers, righting injustices. Geneva, Switzerland: UNAIDS; 2018. doi:10.1111/j.1600-6143.2011.03542 - DOI
-
- UNAIDS. Data 2018. Geneva, Switzerland: UNAIDS; 2018. doi:978-92-9173-945-5
-
- UNICEF. HIV/AIDS data. PMTCT_ARV_2017. 2018https://data.unicef.org/wpcontent/uploads/2018/07/PMTCT_ARV_2017.xlsx (accessed 10 Jun2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

