Elemental bioimaging shows mercury and other toxic metals in normal breast tissue and in breast cancers
- PMID: 32004334
- PMCID: PMC6993973
- DOI: 10.1371/journal.pone.0228226
Elemental bioimaging shows mercury and other toxic metals in normal breast tissue and in breast cancers
Abstract
Objective: Exposure to toxic metals such as mercury has been proposed to be a risk factor for the development of breast cancer since some metals can promote genetic mutations and epigenetic changes. We sought to find what toxic metals are present in normal breast tissue and in the tumours of women who had mastectomies for invasive ductal breast carcinoma.
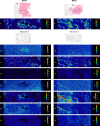
Materials and methods: Formalin-fixed paraffin-embedded blocks from mastectomies for breast carcinoma were examined from 50 women aged 34-69 years. Paraffin blocks selected for elemental analysis were from breast tissue not involved by carcinoma and from the carcinoma itself. Seven micrometer-thick sections were stained with autometallography to demonstrate the presence of mercury, and subjected to laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) to confirm the presence of mercury and to detect other toxic metals.
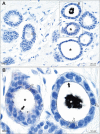
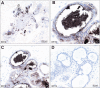
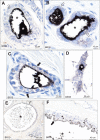
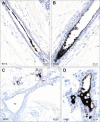
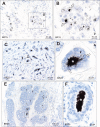
Results: Autometallography-detected mercury was seen in intraductal secretions and some luminal epithelial cells of normal breast lobules in 26 (55%) of the 47 samples where lobules were present, and in 10 (23%) of carcinomas from the 44 samples where carcinoma was present. In eight samples ductal carcinoma in situ was present and one of these contained mercury. LA-ICP-MS confirmed the presence of mercury in samples that stained with autometallography, and detected lead, iron, nickel, aluminium, chromium and cadmium in some samples.
Conclusions: Mercury was present in normal breast lobules in more than half of mastectomy samples that contained an invasive carcinoma, and in a smaller proportion of carcinomas and ductal carcinomas in situ. Other toxic metals that may interact synergistically with mercury could be detected in some samples. These findings do not provide direct evidence that toxic metals such as mercury play a role in the pathogenesis of breast cancer, but suggest that future molecular biological investigations on the role of toxic metals in breast cancer are warranted.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

