The Cellular and Mechanical Basis for Response Characteristics of Identified Primary Afferents in the Rat Vibrissal System
- PMID: 32004452
- PMCID: PMC10623402
- DOI: 10.1016/j.cub.2019.12.068
The Cellular and Mechanical Basis for Response Characteristics of Identified Primary Afferents in the Rat Vibrissal System
Abstract
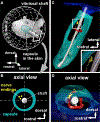
Compared to our understanding of the response properties of receptors in the auditory and visual systems, we have only a limited understanding of the mechanoreceptor responses that underlie tactile sensation. Here, we exploit the stereotyped morphology of the rat vibrissal (whisker) array to investigate coding and transduction properties of identified primary tactile afferents. We performed in vivo intra-axonal recording and labeling experiments to quantify response characteristics of four different types of identified mechanoreceptors in the vibrissal follicle: ring-sinus Merkel; lanceolate; clublike; and rete-ridge collar Merkel. Of these types, only ring-sinus Merkel endings exhibited slowly adapting properties. A weak inverse relationship between response magnitude and onset response latency was found across all types. All afferents exhibited strong "angular tuning," i.e., their response magnitude and latency depended on the whisker's deflection angle. Although previous studies suggested that this tuning should be aligned with the angular location of the mechanoreceptor in the follicle, such alignment was observed only for Merkel afferents; angular tuning of the other afferent types showed no clear alignment with mechanoreceptor location. Biomechanical modeling suggested that this tuning difference might be explained by mechanoreceptors' differential sensitivity to the force directed along the whisker length. Electron microscopic investigations of Merkel endings and lanceolate endings at the level of the ring sinus revealed unique anatomical features that may promote these differential sensitivities. The present study systematically integrates biomechanical principles with the anatomical and morphological characterization of primary afferent endings to describe the physical and cellular processing that shapes the neural representation of touch.
Keywords: 3D reconstruction; active sensing; firing properties; ganglion; in vivo recording; peripheral system; piezoelectric stimulator; rodent; single-cell labeling; trigeminal.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures

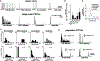



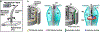
Comment in
-
Somatosensation: The Cellular and Physical Basis of Tactile Experience.Curr Biol. 2020 Mar 9;30(5):R215-R217. doi: 10.1016/j.cub.2020.01.020. Curr Biol. 2020. PMID: 32155422
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

