Global knockout of ROMK potassium channel worsens cardiac ischemia-reperfusion injury but cardiomyocyte-specific knockout does not: Implications for the identity of mitoKATP
- PMID: 32004507
- PMCID: PMC7849919
- DOI: 10.1016/j.yjmcc.2020.01.010
Global knockout of ROMK potassium channel worsens cardiac ischemia-reperfusion injury but cardiomyocyte-specific knockout does not: Implications for the identity of mitoKATP
Abstract
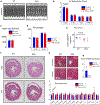
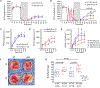
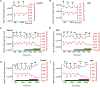
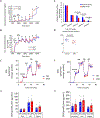
The renal-outer-medullary‑potassium (ROMK) channel, mutated in Bartter's syndrome, regulates ion exchange in kidney, but its extra-renal functions remain unknown. Additionally, ROMK was postulated to be the pore-forming subunit of the mitochondrial ATP-sensitive K+ channel (mitoKATP), a mediator of cardioprotection. Using global and cardiomyocyte-specific knockout mice (ROMK-GKO and ROMK-CKO respectively), we characterize the effects of ROMK knockout on mitochondrial ion handling, the response to pharmacological KATP channel modulators, and ischemia/reperfusion (I/R) injury. Mitochondria from ROMK-GKO hearts exhibited a lower threshold for Ca2+-triggered permeability transition pore (mPTP) opening but normal matrix volume changes during oxidative phosphorylation. Isolated perfused ROMK-GKO hearts exhibited impaired functional recovery and increased infarct size when I/R was preceded by an ischemic preconditioning (IPC) protocol. Because ROMK-GKO mice exhibited severe renal defects and cardiac remodeling, we further characterized ROMK-CKO hearts to avoid confounding systemic effects. Mitochondria from ROMK-CKO hearts had unchanged matrix volume responses during oxidative phosphorylation and still swelled upon addition of a mitoKATP opener, but exhibited a lower threshold for mPTP opening, similar to GKO mitochondria. Nevertheless, I/R induced damage was not exacerbated in ROMK-CKO hearts, either ex vivo or in vivo. Lastly, we examined the response of ROMK-CKO hearts to ex vivo I/R injury with or without IPC and found that IPC still protected these hearts, suggesting that cardiomyocyte ROMK does not participate significantly in the cardioprotective pathway elicited by IPC. Collectively, our findings from these novel strains of mice suggest that cardiomyocyte ROMK is not a central mediator of mitoKATP function, although it can affect mPTP activation threshold.
Keywords: Bartter's syndrome; Ischemic preconditioning; Kcnj1 or Kir1.1 or ROMK; Mitochondrial ATP-sensitive potassium channel; Mitochondrial permeability transition pore; Renal potassium channel.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing interests.
Figures



References
-
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440(7083):470–6. - PubMed
-
- Garlid KD, Paucek P, Yarov-Yarovoy V, Sun X, Schindler PA. The mitochondrial K channel as a receptor for potassium channel openers. Journal of Biological Chemistry. 1996;271(15):8796–9. - PubMed
-
- Liu Y, Sato T, O’Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97(24):2463–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

