IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy
- PMID: 32004713
- PMCID: PMC8173999
- DOI: 10.1016/j.jtho.2020.01.005
IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy
Abstract
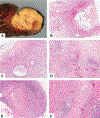
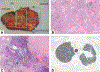
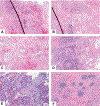
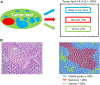
Currently, there is no established guidance on how to process and evaluate resected lung cancer specimens after neoadjuvant therapy in the setting of clinical trials and clinical practice. There is also a lack of precise definitions on the degree of pathologic response, including major pathologic response or complete pathologic response. For other cancers such as osteosarcoma and colorectal, breast, and esophageal carcinomas, there have been multiple studies investigating pathologic assessment of the effects of neoadjuvant therapy, including some detailed recommendations on how to handle these specimens. A comprehensive mapping approach to gross and histologic processing of osteosarcomas after induction therapy has been used for over 40 years. The purpose of this article is to outline detailed recommendations on how to process lung cancer resection specimens and to define pathologic response, including major pathologic response or complete pathologic response after neoadjuvant therapy. A standardized approach is recommended to assess the percentages of (1) viable tumor, (2) necrosis, and (3) stroma (including inflammation and fibrosis) with a total adding up to 100%. This is recommended for all systemic therapies, including chemotherapy, chemoradiation, molecular-targeted therapy, immunotherapy, or any future novel therapies yet to be discovered, whether administered alone or in combination. Specific issues may differ for certain therapies such as immunotherapy, but the grossing process should be similar, and the histologic evaluation should contain these basic elements. Standard pathologic response assessment should allow for comparisons between different therapies and correlations with disease-free survival and overall survival in ongoing and future trials. The International Association for the Study of Lung Cancer has an effort to collect such data from existing and future clinical trials. These recommendations are intended as guidance for clinical trials, although it is hoped they can be viewed as suggestion for good clinical practice outside of clinical trials, to improve consistency of pathologic assessment of treatment response.
Keywords: Lung Cancer; Neoadjuvant therapy; Pathology; Resection specimens; Specimen processing; Treatment response.
Copyright © 2020. Published by Elsevier Inc.
Figures








Comment in
-
Necrosis Is Not the Main Part of Immune-Related Pathologic Response to Neoadjuvant Immunotherapy in Squamous Cell Lung Cancer.J Thorac Oncol. 2021 Jan;16(1):e7-e9. doi: 10.1016/j.jtho.2020.03.032. J Thorac Oncol. 2021. PMID: 33384061 No abstract available.
-
Pathologic Assessment of Lung Squamous Cell Carcinoma After Neoadjuvant Immunotherapy.J Thorac Oncol. 2021 Jan;16(1):e9-e10. doi: 10.1016/j.jtho.2020.11.009. J Thorac Oncol. 2021. PMID: 33384062 No abstract available.
References
-
- Huvos AG, Rosen G, Marcove RC. Primary osteogenic sarcoma: pathologic aspects in 20 patients after treatment with chemotherapy en bloc resection, and prosthetic bone replacement. Arch Pathol Lab Med 1977;101:14–18. - PubMed
-
- Raymond AK, Chawla SP, Carrasco CH, et al. Osteosarcoma chemotherapy effect: a prognostic factor. Seminars in diagnostic pathology 1987;4:212–236. - PubMed
-
- Chui MH, Kandel RA, Wong M, et al. Histopathologic Features of Prognostic Significance in High-Grade Osteosarcoma. Arch Pathol Lab Med 2016;140:1231–1242. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

