Enantioselective [4+2] Annulation to the Concise Synthesis of Chiral Dihydrocarbazoles
- PMID: 32004992
- PMCID: PMC6995259
- DOI: 10.1016/j.isci.2020.100840
Enantioselective [4+2] Annulation to the Concise Synthesis of Chiral Dihydrocarbazoles
Abstract
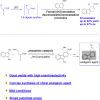
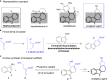
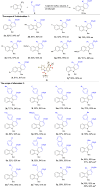
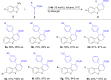
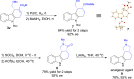
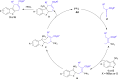
A highly efficient phosphine-catalyzed enantioselective [4 + 2] annulation of allenoates with 3-nitroindoles or 3-nitrobenzothiophenes has been developed. The protocol represents a unique dearomatization-aromatization process to access functionalized dihydrocarbazoles or dihydrodibenzothiophenes with high optical purity (up to 97% ee) under mild reaction conditions. The synthetic utility of the highly enantioselective [4 + 2] annulation enables a concise synthesis of analgesic agent.
Keywords: Catalysis; Organic Reaction; Organic Synthesis.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no conflict of interest.
Figures
References
-
- Awata A., Arai T. Pybidine/Copper catalyst: asymmetric exo’-selective [3+2] cycloaddition using imino ester and electrophilic indole. Angew. Chem. Int. Ed. 2014;53:10462–10465. - PubMed
-
- Baskar B., Dakas P.-Y., Kumar K. Natural product biosynthesis inspired concise and stereoselective synthesis of benzopyrones and related scaffolds. Org. Lett. 2011;13:1988–1991. - PubMed
-
- Carmosin, R.J., Carson, J.R., and Pitis, P.M.. (2000). Octahydropyrrolo-[3,4-C]-carbazoles useful as analgesic agents. US Patent No. 6063803.
LinkOut - more resources
Full Text Sources

