Enhancing panic and smoking reduction treatment with D-Cycloserine: A pilot randomized clinical trial
- PMID: 32004998
- PMCID: PMC7039743
- DOI: 10.1016/j.drugalcdep.2020.107877
Enhancing panic and smoking reduction treatment with D-Cycloserine: A pilot randomized clinical trial
Abstract
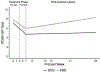
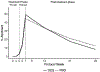
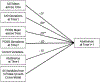
In this placebo-controlled randomized clinical trial, we examined the efficacy of 250 mg d-cycloserine (DCS) for enhancing the effects of cognitive behavior therapy targeting anxiety sensitivity reduction in the context of smoking cessation treatment among adults with a history of panic attacks. We hypothesized that DCS would enhance treatment of our mechanistic targets-anxiety sensitivity and panic and related symptoms-and result in greater smoking abstinence. A total of 53 smokers were randomized to a 7-week integrated treatment and received study medication (DCS or placebo) prior to sessions 3-5; these sessions emphasized interoceptive exposure practice. Nicotine replacement therapy was initiated at session 5 (quit date). We found that DCS augmentation led to greater reductions of one (anxiety sensitivity) of two of our mechanistic targets at early but not late assessments, and that engaging that target predicted better smoking outcomes. However, there was no evidence of group (DCS vs. placebo) differences in smoking cessation success at treatment endpoint or follow-up evaluations. Hence, although we found that DCS can enhance treatment targeting a smoking maintaining factor, additional strategies appear to be needed to significantly affect smoking outcomes.
Trial registration: ClinicalTrials.gov NCT01944423.
Keywords: Anxiety sensitivity; Behavioral intervention; Nicotine-Replacement therapy; Panic; Smoking cessation; d-cycloserine.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Dr. Smits reports receiving support from the National Institutes of Health. He also receives compensation for his work as a consultant to Big Health and his work as editor for Elsevier and the American Psychological Association. He also receives royalties from various book publishers. Dr. Otto reports current and past support from National Institutes of Health. In addition, Dr. Otto reports serving, in the last three years, as a paid speaker and Scientific Advisory Board chair for Big Health, and receiving book royalties from Oxford University Press, Routledge, and Springer. Mr. Papini reports receiving support from the National Institutes of Health and the Donald D. Harrington Foundation.
Figures

References
-
- Bakhshaie J, Kulesz PA, Garey L, Langdon KJ, Businelle MS, Leventhal AM, Gallagher MW, Schmidt NB, Manning K, Goodwin R, Zvolensky MJ, 2018. A prospective investigation of the synergistic effect of change in anxiety sensitivity and dysphoria on tobacco withdrawal. J. Consult. Clin. Psychol 86, 69–80. 10.1037/ccp0000256 - DOI - PMC - PubMed
-
- Bakhshaie J, Zvolensky MJ, Langdon KJ, Leventhal AM, Smits JAJ, Allan N, Schmidt NB, 2016. Anxiety sensitivity class membership moderates the effects of pre-quit reduction in anxiety sensitivity on quit-day tobacco craving. J. Anxiety Disord. 39, 79–87. 10.1016/j.janxdis.2016.02.009 - DOI - PMC - PubMed

