Trauma induced tissue survival in vitro with a muscle-biomaterial based osteogenic organoid system: a proof of concept study
- PMID: 32005149
- PMCID: PMC6995208
- DOI: 10.1186/s12896-020-0602-y
Trauma induced tissue survival in vitro with a muscle-biomaterial based osteogenic organoid system: a proof of concept study
Abstract
Background: The translation from animal research into the clinical environment remains problematic, as animal systems do not adequately replicate the human in vivo environment. Bioreactors have emerged as a good alternative that can reproduce part of the human in vivo processes at an in vitro level. However, in vitro bone formation platforms primarily utilize stem cells only, with tissue based in vitro systems remaining poorly investigated. As such, the present pilot study explored the tissue behavior and cell survival capability within a new in vitro skeletal muscle tissue-based biomaterial organoid bioreactor system to maximize future bone tissue engineering prospects.
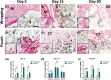
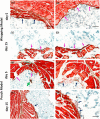
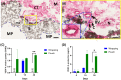
Results: Three dimensional printed β-tricalcium phosphate/hydroxyapatite devices were either wrapped in a sheet of rat muscle tissue or first implanted in a heterotopic muscle pouch that was then excised and cultured in vitro for up to 30 days. Devices wrapped in muscle tissue showed cell death by day 15. Contrarily, devices in muscle pouches showed angiogenic and limited osteogenic gene expression tendencies with consistent TGF-ß1, COL4A1, VEGF-A, RUNX-2, and BMP-2 up-regulation, respectively. Histologically, muscle tissue degradation and fibrin release was seen being absorbed by devices acting possibly as a support for new tissue formation in the bioceramic scaffold that supports progenitor stem cell osteogenic differentiation.
Conclusions: These results therefore demonstrate that the skeletal muscle pouch-based biomaterial culturing system can support tissue survival over a prolonged culture period and represents a novel organoid tissue model that with further adjustments could generate bone tissue for direct clinical transplantations.
Keywords: 3D printed β-TCP/HA; Angiogenesis; Heterotopic implant model; In vitro; Organoid; Pilot study; Tissue survival; Vasculogenesis.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures




References
-
- Denayer T, Stöhr T, Van Roy M. Animal models in translational medicine: validation and prediction. New Horiz Transl Med. 2014;2(1):5–11.
-
- Havers C, Geuder MF. Osteologia nova: sive, Novae quaedam observationes de ossibus, et partibus ad illa pertinentibus: Apud Georgium Wilhelmum Kühnium. 1692.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

