Does vancomycin resistance increase mortality in Enterococcus faecium bacteraemia after orthotopic liver transplantation? A retrospective study
- PMID: 32005223
- PMCID: PMC6995054
- DOI: 10.1186/s13756-020-0683-3
Does vancomycin resistance increase mortality in Enterococcus faecium bacteraemia after orthotopic liver transplantation? A retrospective study
Abstract
Background: The relevance of vancomycin resistance in enterococcal blood stream infections (BSI) is still controversial. Aim of this study was to outline the effect of vancomycin resistance of Enterococcus faecium on the outcome of patients with BSI after orthotopic liver transplantation (OLT).
Methods: The outcome of OLT recipients developing BSI with vancomycin-resistant (VRE) versus vancomycin-susceptible Enterococcus faecium (VSE) was compared based on data extraction from medical records. Multivariate regression analyses identified risk factors for mortality and unfavourable outcomes (defined as death or prolonged intensive care stay) after 30 and 90 days.
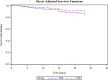
Results: Mortality was similar between VRE- (n = 39) and VSE- (n = 138) group after 30 (p = 0.44) or 90 days (p = 0.39). Comparable results occurred regarding unfavourable outcomes. Mean SOFANon-GCS score during the 7-day-period before BSI onset was the independent predictor for mortality at both timepoints (HR 1.32; CI 1.14-1.53; and HR 1.18; CI 1.08-1.28). Timely appropriate antibiotic therapy, recent ICU stay and vancomycin resistance did not affect outcome after adjusting for confounders.
Conclusion: Vancomycin resistance did not influence outcome among patients with Enterococcus faecium bacteraemia after OLT. Only underlying severity of disease predicted poor outcome among this homogenous patient population.
Trial registration: This study was registered at the German clinical trials register (DRKS-ID: DRKS00013285).
Keywords: Bacteraemia; Enterococci; Liver transplantation; Mortality; Vancomycin resistance.
Conflict of interest statement
Karl-Heinz Weiss advises for Alexion, Univar, GMP-O, Ipsen, Chiesi, Novartis. Karl-Heinz Weiss is on the speaker’s bureau of Pfizer, Abbvie, Eisai. Karl-Heinz Weiss received grant support (to the institution) from AstraZenica, QED, Novartis, Univar, Alexion and GMP-O. Markus Weigand is on the advisory boards from MSD, Gilead, Pfizer, Shionogi. Thorsten Brenner received research funding from German Research Foundation (DFG), Heidelberg Foundation of Surgery, Dietmar Hopp Foundation. Thorsten Brenner received honoraria for lectures and advisory boards from: Biotest AG, Baxter Deutschland GmbH, Schöchl medical education GmbH, Boehringer Ingelheim Pharma GmbH, CSL Behring GmbH, Astellas Pharma GmbH, B. Braun Melsungen AG, MSD Sharp & Dohme GmbH. Alexandra Heininger received speaker honorary by MSD and Pfizer. Simon Dubler, Martin Lenz, Stefan Zimmermann, Daniel Richter, Arianeb Mehrabi, Markus Mieth and Thomas Brucker declare that they have no competing interests.
Figures
Similar articles
-
The importance of adjusting for enterococcus species when assessing the burden of vancomycin resistance: a cohort study including over 1000 cases of enterococcal bloodstream infections.Antimicrob Resist Infect Control. 2018 Nov 14;7:133. doi: 10.1186/s13756-018-0419-9. eCollection 2018. Antimicrob Resist Infect Control. 2018. PMID: 30459945 Free PMC article.
-
Impact of Enterococcal Bacteremia in Liver Transplant Recipients.Transplant Proc. 2019 Oct;51(8):2766-2770. doi: 10.1016/j.transproceed.2019.02.064. Epub 2019 Sep 4. Transplant Proc. 2019. PMID: 31493916
-
Impact of vancomycin resistance on mortality in neutropenic patients with enterococcal bloodstream infection: a retrospective study.BMC Infect Dis. 2013 Oct 29;13:504. doi: 10.1186/1471-2334-13-504. BMC Infect Dis. 2013. PMID: 24164924 Free PMC article.
-
Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis.Clin Infect Dis. 2005 Aug 1;41(3):327-33. doi: 10.1086/430909. Epub 2005 Jun 28. Clin Infect Dis. 2005. PMID: 16007529
-
Treatment options for vancomycin-resistant enterococcal infections.Drugs. 2002;62(3):425-41. doi: 10.2165/00003495-200262030-00002. Drugs. 2002. PMID: 11827558 Review.
Cited by
-
Vancomycin resistant enterococcus risk factors for hospital colonization in hematological patients: a matched case-control study.Antimicrob Resist Infect Control. 2023 Nov 13;12(1):126. doi: 10.1186/s13756-023-01332-x. Antimicrob Resist Infect Control. 2023. PMID: 37957773 Free PMC article.
-
The Prevalence of Alert Pathogens and Microbial Resistance Mechanisms: A Three-Year Retrospective Study in a General Hospital in Poland.Pathogens. 2023 Nov 28;12(12):1401. doi: 10.3390/pathogens12121401. Pathogens. 2023. PMID: 38133286 Free PMC article.
-
Multidrug-resistant organism bloodstream infections in solid organ transplant recipients and impact on mortality: a systematic review.JAC Antimicrob Resist. 2024 Oct 9;6(5):dlae152. doi: 10.1093/jacamr/dlae152. eCollection 2024 Oct. JAC Antimicrob Resist. 2024. PMID: 39386374 Free PMC article. Review.
-
Impact of vancomycin area under the curve in early or later phase on efficacy and nephrotoxicity in patients with enterococcal bloodstream infections: a multicenter study.BMC Infect Dis. 2025 Jan 28;25(1):133. doi: 10.1186/s12879-024-10399-9. BMC Infect Dis. 2025. PMID: 39875832 Free PMC article.
-
Effect of Dexamethasone on the Incidence and Outcome of COVID-19 Associated Pulmonary Aspergillosis (CAPA) in Critically Ill Patients during First- and Second Pandemic Wave-A Single Center Experience.Diagnostics (Basel). 2022 Dec 5;12(12):3049. doi: 10.3390/diagnostics12123049. Diagnostics (Basel). 2022. PMID: 36553055 Free PMC article.
References
-
- Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34(1):1–14. doi: 10.1086/668770. - DOI - PubMed
-
- Tacconelli E, Magrini N. GLOBAL PRIORITY LIST OF ANTIBIOTIC-RESISTANT BACTERIA TO GUIDE RESEARCH, DISCOVERY, AND DEVELOPMENT OF NEW ANTIBIOTICS. World Health Organization (WHO). 2017:7.
-
- (ECDC) ECfDPaC. Annual epidemiological report for 2016 - Healthcare-associated infections acquired in intensivecare units. ECDC Annual epidemiological report for 2016. 2018.
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

