Choline supplementation prevents diet induced gut mucosa lipid accumulation in post-smolt Atlantic salmon (Salmo salar L.)
- PMID: 32005242
- PMCID: PMC6995171
- DOI: 10.1186/s12917-020-2252-7
Choline supplementation prevents diet induced gut mucosa lipid accumulation in post-smolt Atlantic salmon (Salmo salar L.)
Abstract
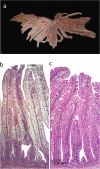
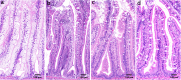
Background: Various intestinal morphological alterations have been reported in cultured fish fed diets with high contents of plant ingredients. Since 2000, salmon farmers have reported symptoms indicating an intestinal problem, which we suggest calling lipid malabsorption syndrome (LMS), characterized by pale and foamy appearance of the enterocytes of the pyloric caeca, the result of lipid accumulation. The objective of the present study was to investigate if insufficient dietary choline may be a key component in development of the LMS.
Results: The results showed that Atlantic salmon (Salmo salar), average weight 362 g, fed a plant based diet for 79 days developed signs of LMS. In fish fed a similar diet supplemented with 0.4% choline chloride no signs of LMS were seen. The relative weight of the pyloric caeca was 40% lower, reflecting 65% less triacylglycerol content and histologically normal gut mucosa. Choline supplementation further increased specific fish growth by 18%. The concomitant alterations in intestinal gene expression related to phosphatidylcholine synthesis (chk and pcyt1a), cholesterol transport (abcg5 and npc1l1), lipid metabolism and transport (mgat2a and fabp2) and lipoprotein formation (apoA1 and apoAIV) confirmed the importance of choline in lipid turnover in the intestine and its ability to prevent LMS. Another important observation was the apparent correlation between plin2 expression and degree of enterocyte hyper-vacuolation observed in the current study, which suggests that plin2 may serve as a marker for intestinal lipid accumulation and steatosis in fish. Future research should be conducted to strengthen the knowledge of choline's critical role in lipid transport, phospholipid synthesis and lipoprotein secretion to improve formulations of plant based diets for larger fish and to prevent LMS.
Conclusions: Choline prevents excessive lipid accumulation in the proximal intestine and is essential for Atlantic salmon in seawater.
Keywords: Choline; Fish feed; Gut health; LMS; Lipid accumulation; Lipid malabsorption; Lipid transport; Plant ingredients.
Conflict of interest statement
The present study was partly funded by BioMar AS. Co-author Anne Kristine Hansen is employed by BioMar.
Figures




References
-
- NRC . Nutrient Requirements of Fish and Shrimp. Washingt DC: Natl Acad Press; 2011. pp. 102–134.
-
- Hanche-Olsen R, Brunvold L, Hillestad M, Lysne H, Penn M, Løland A. Sluttrapport: Nedsatt Tarmhelse Og Forekomst Av Flytefeces Hos Laks (In Norwegian) 2013.
-
- Penn M. Lipid malabsorption in Atlantic Salmon – the recurring problem of floating feces. 2011. pp. 6–11.
-
- Elvis M, Chikwati YL, Wang J, Zhou W, Hage E, Præsteng K, Kortner TM, Torres AJ, Gajardo K, Løkka G, Aru V, Khakimov B, Engelsen SB, Bjørgen H, Koppang EO, Gerd AK. 8th International Symposium on Aquatic Animal Health. 2018. Gut health monitoring during the seawater phase of farmed Atlantic salmon in different production regions of Norway – the GutMatters project.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

