Isolation of Ontario aquatic bird bornavirus 1 and characterization of its replication in immortalized avian cell lines
- PMID: 32005267
- PMCID: PMC6995091
- DOI: 10.1186/s12985-020-1286-6
Isolation of Ontario aquatic bird bornavirus 1 and characterization of its replication in immortalized avian cell lines
Abstract
Background: Aquatic bird bornavirus 1 (ABBV-1) has been associated with neurological diseases in wild waterfowls. In Canada, presence of ABBV-1 was demonstrated by RT-qPCR and immunohistochemistry in tissues of waterfowls with history of neurological disease and inflammation of the central and peripheral nervous tissue, although causation has not been proven by pathogenesis experiments, yet. To date, in vitro characterization of ABBV-1 is limited to isolation in primary duck embryo fibroblasts. The objectives of this study were to describe isolation of ABBV-1 in primary duck embryonic fibroblasts (DEF), and characterize replication in DEF and three immortalized avian fibroblast cell lines (duck CCL-141, quail QT-35, chicken DF-1) in order to evaluate cellular permissivity and identify suitable cell lines for routine virus propagation.
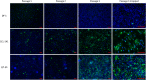
Methods: The virus was sequenced, and phylogenetic analysis performed on a segment of the N gene coding region. Virus spread in cell cultures, viral RNA and protein production, and titres were evaluated at different passages using immunofluorescence, RT-qPCR, western blotting, and tissue culture dose 50% (TCID50) assay, respectively.
Results: The isolated ABBV-1 showed 97 and 99% identity to European ABBV-1 isolate AF-168 and North American ABBV-1 isolates 062-CQ and CG-N1489, and could infect and replicate in DEF, CCL-141, QT-35 and DF-1 cultures. Viral RNA was detected in all four cultures with highest levels observed in DEF and CCL-141, moderate in QT-35, and lowest in DF-1. N protein was detected in western blots from infected DEF, CCL-141 and QT-35 at moderate to high levels, but minimally in infected DF-1. Infectious titre was highest in DEF (between approximately 105 to 106 FFU / 106 cells). Regarding immortalized cell lines, CCL-141 showed the highest titre between approximately 104 to 105 FFU / 106 cells. DF-1 produced minimal infectious titre.
Conclusions: This study confirms the presence of ABBV-1 among waterfowl in Canada and reported additional in vitro characterization of this virus in different avian cell lines. ABBV-1 replicated to highest titre in DEF, followed by CCL-141 and QT-35, and poorly in DF-1. Our results showed that CCL-141 can be used instead of DEF for routine ABBV-1 production, if a lower titre is an acceptable trade-off for the simplicity of using immortalized cell line over primary culture.
Keywords: ABBV-1 replication in avian cells; Aquatic bird bornavirus-1; Avian bornavirus; Immortalized avian cell lines, duck embryo fibroblasts; Persistent infection.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

