Allogeneic human umbilical cord-derived mesenchymal stem cells for severe bronchopulmonary dysplasia in children: study protocol for a randomized controlled trial (MSC-BPD trial)
- PMID: 32005282
- PMCID: PMC6995070
- DOI: 10.1186/s13063-019-3935-x
Allogeneic human umbilical cord-derived mesenchymal stem cells for severe bronchopulmonary dysplasia in children: study protocol for a randomized controlled trial (MSC-BPD trial)
Abstract
Background: Bronchopulmonary dysplasia (BPD) is a complex lung pathological lesion secondary to multiple factors and one of the most common chronic lung diseases. It has a poor prognosis, especially in preterm infants. However, effective therapies for this disease are lacking. Stem-cell therapy is a promising way to improve lung injury and abnormal alveolarization, and the human umbilical cord (hUC) is a good source of mesenchymal stem cells (MSCs), which have demonstrated efficacy in other diseases. We hypothesized that intravenously administered allogeneic hUC-MSCs are safe and effective for severe BPD.
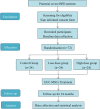
Methods: The MSC-BPD trial is a randomized, single-center, open-label, dose-escalation, phase-II trial designed to investigate the safety and efficacy of hUC-MSCs in children with severe BPD. In this study, 72 patients will be enrolled and randomly divided into two intervention groups and one control group. Patients in the intervention groups will receive a low dose of hUC-MSCs (n = 24; 2.5 million cells/kg) or a high dose of hUC-MSCs (n = 24; 5 million cells/kg) in combination with traditional supportive treatments for BPD. The patients in the control group (n = 24) will be treated with traditional supportive treatments alone without hUC-MSCs. The primary outcome measures will be cumulative duration of oxygen therapy. Follow-up assessments will be performed at 1, 3, 6, 12, and 24 months post intervention, and the key outcome during follow-up will be changes on chest radiography. Statistical analyses will evaluate the efficacy of the hUC-MSC treatment.
Discussion: This will be the first randomized controlled trial to evaluate the safety and efficacy of intravenously administered hUC-MSCs in children with severe BPD. Its results should provide a new evidence-based therapy for severe BPD.
Trial registration: ClinicalTrials.gov, ID: NCT03601416. Registered on 26 July 2018.
Keywords: Bronchopulmonary dysplasia; Clinical trial; Human umbilical cord-derived mesenchymal stem cells; Protocol.
Conflict of interest statement
The authors declare that they have no competing interests.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical