Effect of Daesiho-tang on obesity with non-alcoholic fatty liver disease: a study protocol for a randomised, double-blind, placebo-controlled pilot trial
- PMID: 32005283
- PMCID: PMC6995056
- DOI: 10.1186/s13063-020-4068-y
Effect of Daesiho-tang on obesity with non-alcoholic fatty liver disease: a study protocol for a randomised, double-blind, placebo-controlled pilot trial
Abstract
Background: The high prevalence of obesity and non-alcoholic fatty acid disease has become an important public health problem. Daesiho-tang (DST) is an herbal medicine widely used to treat obesity, metabolic syndrome and liver diseases. This pilot study will assess the feasibility of using DST in obese patients with a non-alcoholic fatty liver disease (NAFLD) prior to undertaking a full-scale clinical trial.
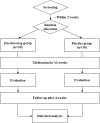
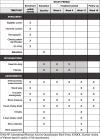
Methods/design: This is a study protocol for a randomised, double-blind, parallel-group, stratified, placebo-controlled pilot trial. We will recruit a total of 60 participants with NAFLD who have a body mass index ≥ 25 kg/m2. They will take either DST or placebo (3 g, three times daily) for 12 weeks with a 4-week follow-up period. The effects of DST will be evaluated by the mean change in body weight as the primary measurement and other secondary parameters (body composition, anthropometric measurements, blood tests, hepatic fat quantification through transient elastography and a physical symptoms questionnaire). Faecal samples will be collected before and after the intervention for a gut microbial analysis.
Discussion: In anticipation of conducting further large-scale trials, in this study we will explore the effect of DST on weight loss and obesity-related markers, along with NAFLD-related clinical parameters, in obese patients with NAFLD. Furthermore, it will provide insight into the DST pharmacological mechanism of action through a gut microbiome analysis.
Trial registration: Korean Clinical Trial Registry, KCT0003554. Registered on 25 February 2019.
Keywords: Dachaihu-tang; Daesiho-tang; Dai-saiko-to; Herbal medicine; Non-alcoholic fatty liver disease; Obesity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical