Structure-function relationships of the soluble form of the antiaging protein Klotho have therapeutic implications for managing kidney disease
- PMID: 32005658
- PMCID: PMC7062171
- DOI: 10.1074/jbc.RA119.012144
Structure-function relationships of the soluble form of the antiaging protein Klotho have therapeutic implications for managing kidney disease
Abstract
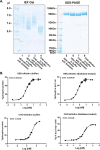
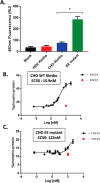
The fortuitously discovered antiaging membrane protein αKlotho (Klotho) is highly expressed in the kidney, and deletion of the Klotho gene in mice causes a phenotype strikingly similar to that of chronic kidney disease (CKD). Klotho functions as a co-receptor for fibroblast growth factor 23 (FGF23) signaling, whereas its shed extracellular domain, soluble Klotho (sKlotho), carrying glycosidase activity, is a humoral factor that regulates renal health. Low sKlotho in CKD is associated with disease progression, and sKlotho supplementation has emerged as a potential therapeutic strategy for managing CKD. Here, we explored the structure-function relationship and post-translational modifications of sKlotho variants to guide the future design of sKlotho-based therapeutics. Chinese hamster ovary (CHO)- and human embryonic kidney (HEK)-derived WT sKlotho proteins had varied activities in FGF23 co-receptor and β-glucuronidase assays in vitro and distinct properties in vivo Sialidase treatment of heavily sialylated CHO-sKlotho increased its co-receptor activity 3-fold, yet it remained less active than hyposialylated HEK-sKlotho. MS and glycopeptide-mapping analyses revealed that HEK-sKlotho is uniquely modified with an unusual N-glycan structure consisting of N,N'-di-N-acetyllactose diamine at multiple N-linked sites, one of which at Asn-126 was adjacent to a putative GalNAc transfer motif. Site-directed mutagenesis and structural modeling analyses directly implicated N-glycans in Klotho's protein folding and function. Moreover, the introduction of two catalytic glutamate residues conserved across glycosidases into sKlotho enhanced its glucuronidase activity but decreased its FGF23 co-receptor activity, suggesting that these two functions might be structurally divergent. These findings open up opportunities for rational engineering of pharmacologically enhanced sKlotho therapeutics for managing kidney disease.
Keywords: Klotho; LacdiNAc; acute kidney injury; fibroblast growth factor receptor (FGFR); glycosidase; glycosylation; mammalian cell expression; pharmacokinetics; sialic acid; signal transduction.
© 2020 Zhong et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Levey A. S., Atkins R., Coresh J., Cohen E. P., Collins A. J., Eckardt K. U., Nahas M. E., Jaber B. L., Jadoul M., Levin A., Powe N. R., Rossert J., Wheeler D. C., Lameire N., and Eknoyan G. (2007) Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 72, 247–259 10.1038/sj.ki.5002343 - DOI - PubMed
-
- Radhakrishnan J., Remuzzi G., Saran R., Williams D. E., Rios-Burrows N., Powe N., CDC-CKD Surveillance Team, Brück K., Wanner C., Stel V. S., European CKD Burden Consortium, Venuthurupalli S. K., Hoy W. E., Healy H. G., Salisbury A., et al. (2014) Taming the chronic kidney disease epidemic: a global view of surveillance efforts. Kidney Int. 86, 246–250 10.1038/ki.2014.190 - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

