Exploring Cryptococcus neoformans capsule structure and assembly with a hydroxylamine-armed fluorescent probe
- PMID: 32005661
- PMCID: PMC7105310
- DOI: 10.1074/jbc.RA119.012251
Exploring Cryptococcus neoformans capsule structure and assembly with a hydroxylamine-armed fluorescent probe
Abstract
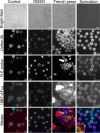
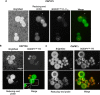
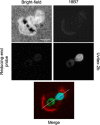
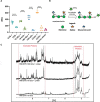
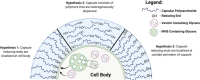
Chemical biology is an emerging field that enables the study and manipulation of biological systems with probes whose reactivities provide structural insights. The opportunistic fungal pathogen Cryptococcus neoformans possesses a polysaccharide capsule that is a major virulence factor, but is challenging to study. We report here the synthesis of a hydroxylamine-armed fluorescent probe that reacts with reducing glycans and its application to study the architecture of the C. neoformans capsule under a variety of conditions. The probe signal localized intracellularly and at the cell wall-membrane interface, implying the presence of reducing-end glycans at this location where the capsule is attached to the cell body. In contrast, no fluorescence signal was detected in the capsule body. We observed vesicle-like structures containing the reducing-end probe, both intra- and extracellularly, consistent with the importance of vesicles in capsular assembly. Disrupting the capsule with DMSO, ultrasound, or mechanical shear stress resulted in capsule alterations that affected the binding of the probe, as reducing ends were exposed and cell membrane integrity was compromised. Unlike the polysaccharides in the assembled capsule, isolated exopolysaccharides contained reducing ends. The reactivity of the hydroxylamine-armed fluorescent probe suggests a model for capsule assembly whereby reducing ends localize to the cell wall surface, supporting previous findings suggesting that this is an initiation point for capsular assembly. We propose that chemical biology is a promising approach for studying the C. neoformans capsule and its associated polysaccharides to unravel their roles in fungal virulence.
Keywords: aminooxy fluorescent probes; biosynthesis; capsule; chemical biology; extracellular vesicles; fungal pathogen; fungi; glucuronoxylomannan; glycobiology; polysaccharide; reducing glycans; vesicles; virulence factor.
© 2020 Crawford et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




Similar articles
-
Cryptococcus neoformans capsule regrowth experiments reveal dynamics of enlargement and architecture.J Biol Chem. 2022 Apr;298(4):101769. doi: 10.1016/j.jbc.2022.101769. Epub 2022 Feb 24. J Biol Chem. 2022. PMID: 35218774 Free PMC article.
-
Cryptococcus neoformans capsular polysaccharides form branched and complex filamentous networks viewed by high-resolution microscopy.J Struct Biol. 2016 Jan;193(1):75-82. doi: 10.1016/j.jsb.2015.11.010. Epub 2015 Nov 30. J Struct Biol. 2016. PMID: 26655746
-
Pbx proteins in Cryptococcus neoformans cell wall remodeling and capsule assembly.Eukaryot Cell. 2014 May;13(5):560-71. doi: 10.1128/EC.00290-13. Epub 2014 Feb 28. Eukaryot Cell. 2014. PMID: 24585882 Free PMC article.
-
The Overlooked Glycan Components of the Cryptococcus Capsule.Curr Top Microbiol Immunol. 2019;422:31-43. doi: 10.1007/82_2018_140. Curr Top Microbiol Immunol. 2019. PMID: 30203395 Review.
-
The capsule of the fungal pathogen Cryptococcus neoformans.Adv Appl Microbiol. 2009;68:133-216. doi: 10.1016/S0065-2164(09)01204-0. Adv Appl Microbiol. 2009. PMID: 19426855 Free PMC article. Review.
Cited by
-
A synthetic glycan array containing Cryptococcus neoformans glucuronoxylomannan capsular polysaccharide fragments allows the mapping of protective epitopes.Chem Sci. 2020 Aug 7;11(34):9209-9217. doi: 10.1039/d0sc01249a. Chem Sci. 2020. PMID: 34123169 Free PMC article.
-
Minimal domain peptides derived from enterocins exhibit potent antifungal activity.Front Fungal Biol. 2024 Dec 19;5:1506315. doi: 10.3389/ffunb.2024.1506315. eCollection 2024. Front Fungal Biol. 2024. PMID: 39749139 Free PMC article.
-
Synthetic Glycans Reveal Determinants of Antibody Functional Efficacy against a Fungal Pathogen.ACS Infect Dis. 2024 Feb 9;10(2):475-488. doi: 10.1021/acsinfecdis.3c00447. Epub 2023 Oct 19. ACS Infect Dis. 2024. PMID: 37856427 Free PMC article.
-
A glycan FRET assay for detection and characterization of catalytic antibodies to the Cryptococcus neoformans capsule.Proc Natl Acad Sci U S A. 2021 Feb 2;118(5):e2016198118. doi: 10.1073/pnas.2016198118. Proc Natl Acad Sci U S A. 2021. PMID: 33514659 Free PMC article.
-
Variation in Cell Surface Hydrophobicity among Cryptococcus neoformans Strains Influences Interactions with Amoebas.mSphere. 2020 Apr 29;5(2):e00310-20. doi: 10.1128/mSphere.00310-20. mSphere. 2020. PMID: 32350094 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

