Serine phosphorylation of the small phosphoprotein ICAP1 inhibits its nuclear accumulation
- PMID: 32005669
- PMCID: PMC7062153
- DOI: 10.1074/jbc.RA119.009794
Serine phosphorylation of the small phosphoprotein ICAP1 inhibits its nuclear accumulation
Abstract
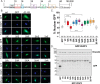
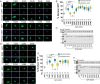
Nuclear accumulation of the small phosphoprotein integrin cytoplasmic domain-associated protein-1 (ICAP1) results in recruitment of its binding partner, Krev/Rap1 interaction trapped-1 (KRIT1), to the nucleus. KRIT1 loss is the most common cause of cerebral cavernous malformation, a neurovascular dysplasia resulting in dilated, thin-walled vessels that tend to rupture, increasing the risk for hemorrhagic stroke. KRIT1's nuclear roles are unknown, but it is known to function as a scaffolding or adaptor protein at cell-cell junctions and in the cytosol, supporting normal blood vessel integrity and development. As ICAP1 controls KRIT1 subcellular localization, presumably influencing KRIT1 function, in this work, we investigated the signals that regulate ICAP1 and, hence, KRIT1 nuclear localization. ICAP1 contains a nuclear localization signal within an unstructured, N-terminal region that is rich in serine and threonine residues, several of which are reportedly phosphorylated. Using quantitative microscopy, we revealed that phosphorylation-mimicking substitutions at Ser-10, or to a lesser extent at Ser-25, within this N-terminal region inhibit ICAP1 nuclear accumulation. Conversely, phosphorylation-blocking substitutions at these sites enhanced ICAP1 nuclear accumulation. We further demonstrate that p21-activated kinase 4 (PAK4) can phosphorylate ICAP1 at Ser-10 both in vitro and in cultured cells and that active PAK4 inhibits ICAP1 nuclear accumulation in a Ser-10-dependent manner. Finally, we show that ICAP1 phosphorylation controls nuclear localization of the ICAP1-KRIT1 complex. We conclude that serine phosphorylation within the ICAP1 N-terminal region can prevent nuclear ICAP1 accumulation, providing a mechanism that regulates KRIT1 localization and signaling, potentially influencing vascular development.
Keywords: ICAP1 (integrin cytoplasmic domain-associated protein-1); KRIT1 (Krev interaction trapped); cell compartmentalization; cerebral cavernous malformation; nuclear import; nuclear translocation; nuclear transport; nucleocytoplasmic shuttling; phosphoprotein; phosphorylation.
© 2020 Su et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures








References
-
- Faurobert E., Rome C., Lisowska J., Manet-Dupé S., Boulday G., Malbouyres M., Balland M., Bouin A.-P., Kéramidas M., Bouvard D., Coll J.-L., Ruggiero F., Tournier-Lasserve E., and Albiges-Rizo C. (2013) CCM1–ICAP-1 complex controls β1 integrin–dependent endothelial contractility and fibronectin remodeling. J. Cell Biol. 202, 545–561 10.1083/jcb.201303044 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

