Risk factors influencing contamination of customized cosmetics made on-the-spot: Evidence from the national pilot project for public health
- PMID: 32005845
- PMCID: PMC6994525
- DOI: 10.1038/s41598-020-57978-9
Risk factors influencing contamination of customized cosmetics made on-the-spot: Evidence from the national pilot project for public health
Erratum in
-
Author Correction: Risk factors influencing contamination of customized cosmetics made on-the-spot: Evidence from the national pilot project for public health.Sci Rep. 2020 Apr 30;10(1):7587. doi: 10.1038/s41598-020-63867-y. Sci Rep. 2020. PMID: 32350299 Free PMC article.
Abstract
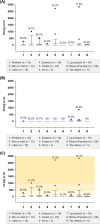
Customized cosmetics made by consumers or sellers on-the-spot have several safety issues, and therefore require a preventative approach to their safety management. The present study aimed to identify potential factors affecting the safety of customized cosmetics made on-the-spot. Heavy metals and microbial contaminants in customized cosmetics were analyzed in 120 samples. It was revealed that the transfer of cosmetics to new containers during the production process is a significant risk factor for cross-contamination and that heat treatment is crucial for reducing the number of microorganisms in the products. For instance, cosmetics made with heat and with no transfer showed relatively low microbial counts ranging from not detected to 440 CFU/ml. The high pH (>pH 10) of samples did not guarantee the microbial safety of the freshly made cosmetics (with a rinse-off product having 2,830 CFU/ml and a pH of 11.2). There was no significant difference in microbial counts among cosmetic types (P > 0.05); however, semisolid types, especially creams and rinse-off products, were susceptible to contamination (maximum 2,710 and 2,830 CFU/ml, respectively). Most microorganisms in the customized cosmetics (40.8%) decreased to non-detectable levels during 60 days of storage. None of the samples harbored heavy metals. Sequencing analysis of isolates revealed some bacteria and mold that could cause human infections. The results of this study suggest that the regulation of customized cosmetics should consider the risk factors revealed in this study, as the products made on-the-spot are also final products sent directly to consumers.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- International Trade Administration, Korea Country Commercial Guide-Cosmetics (available at, https://www.export.gov/article?id=Korea-Cosmetics), (2017).
-
- Linaja, K.L., New trend: Korean customized cosmetics gains popularity (available at, http://en.koreaportal.com/articles/22227/20160907/new-trend-korean-custo...) (2016).
-
- Lombardi, J. & Morante, N., Method and system for color customizing cosmetic mass products, US Patent No. 6,177,093 (2001).
-
- O’Brien, M. Customizing cosmetics: Why personalization is especially important in the beauty industry (available at, https://www.sailthru.com/marketing-blog/personalization-beauty-industry/) (2018).
-
- Rhee, M.S. et al, Improvement plan of customized cosmetics management system, Government report from Ministry of Food and Drug Safety in Korea 15162MFDS053 (2016).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

