Hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitors induce autophagy and have a protective effect in an in-vitro ischaemia model
- PMID: 32005890
- PMCID: PMC6994562
- DOI: 10.1038/s41598-020-58482-w
Hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitors induce autophagy and have a protective effect in an in-vitro ischaemia model
Erratum in
-
Author Correction: Hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitors induce autophagy and have a protective effect in an in-vitro ischaemia model.Sci Rep. 2020 Apr 8;10(1):6041. doi: 10.1038/s41598-020-63108-2. Sci Rep. 2020. PMID: 32269283 Free PMC article.
Abstract
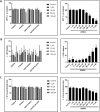
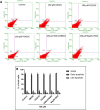
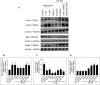
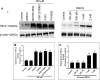
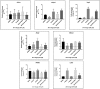
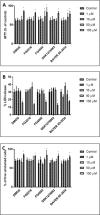
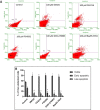
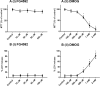
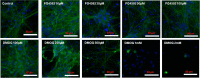
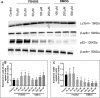
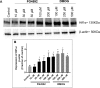
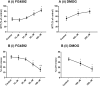
This study compared effects of five hypoxia-inducible factor (HIF) prolyl hydroxylases (PHD) inhibitors on PC12 cells and primary rat neurons following oxygen-glucose deprivation (OGD). At 100 µM, the PHD inhibitors did not cause cytotoxicity and apoptosis. MTT activity was only significantly reduced by FG4592 or Bayer 85-3934 in PC12 cells. The PHD inhibitors at 100 µM significantly increased the LC3-II/LC3-I expression ratio and downregulated p62 in PC12 cells, so did FG4592 (30 µM) and DMOG (100 µM) in neurons. HIF-1α was stabilised in PC12 cells by all the PHD inhibitors at 100 µM except for DMOG, which stabilised HIF-1α at 1 and 2 mM. In primary neurons, HIF-1α was stabilised by FG4592 (30 µM) and DMOG (100 µM). Pretreatment with the PHD inhibitors 24 hours followed by 24 hour reoxygenation prior to 6 hours OGD (0.3% O2) significantly reduced LDH release and increased MTT activity compared to vehicle (1% DMSO) pretreatment. In conclusion, the PHD inhibitors stabilise HIF-1α in normoxia, induce autophagy, and protect cells from a subsequent OGD insult. The new class of PHD inhibitors (FG4592, FG2216, GSK1278863, Bay85-3934) have the higher potency than DMOG. The interplay between autophagy, HIF stabilisation and neuroprotection in ischaemic stroke merits further investigation.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Effects of prolyl-hydroxylase inhibition and chronic intermittent hypoxia on synaptic transmission and plasticity in the rat CA1 and dentate gyrus.Neurobiol Dis. 2014 Feb;62:8-17. doi: 10.1016/j.nbd.2013.08.016. Epub 2013 Sep 17. Neurobiol Dis. 2014. PMID: 24055213
-
Inhibition of prolyl hydroxylases by dimethyloxaloylglycine after stroke reduces ischemic brain injury and requires hypoxia inducible factor-1α.Neurobiol Dis. 2012 Feb;45(2):733-42. doi: 10.1016/j.nbd.2011.10.020. Epub 2011 Oct 29. Neurobiol Dis. 2012. PMID: 22061780 Free PMC article.
-
Erythropoietin inhibits HIF-1α expression via upregulation of PHD-2 transcription and translation in an in vitro model of hypoxia-ischemia.Transl Stroke Res. 2014 Feb;5(1):118-27. doi: 10.1007/s12975-013-0312-z. Epub 2013 Nov 27. Transl Stroke Res. 2014. PMID: 24323731 Free PMC article.
-
Protein Hydroxylation by Hypoxia-Inducible Factor (HIF) Hydroxylases: Unique or Ubiquitous?Cells. 2019 Apr 26;8(5):384. doi: 10.3390/cells8050384. Cells. 2019. PMID: 31035491 Free PMC article. Review.
-
Recent Advances in Developing Inhibitors for Hypoxia-Inducible Factor Prolyl Hydroxylases and Their Therapeutic Implications.Molecules. 2015 Nov 19;20(11):20551-68. doi: 10.3390/molecules201119717. Molecules. 2015. PMID: 26610437 Free PMC article. Review.
Cited by
-
The Duration of Oxygen and Glucose Deprivation (OGD) Determines the Effects of Subsequent Reperfusion on Rat Pheochromocytoma (PC12) Cells and Primary Cortical Neurons.Int J Mol Sci. 2023 Apr 12;24(8):7106. doi: 10.3390/ijms24087106. Int J Mol Sci. 2023. PMID: 37108268 Free PMC article.
-
Hypoxia, HIF-1α, and COVID-19: from pathogenic factors to potential therapeutic targets.Acta Pharmacol Sin. 2020 Dec;41(12):1539-1546. doi: 10.1038/s41401-020-00554-8. Epub 2020 Oct 27. Acta Pharmacol Sin. 2020. PMID: 33110240 Free PMC article. Review.
-
Pharmacological strategies for treating misfolded rhodopsin-associated autosomal dominant retinitis pigmentosa.Neural Regen Res. 2022 Jan;17(1):110-112. doi: 10.4103/1673-5374.314306. Neural Regen Res. 2022. PMID: 34100444 Free PMC article. No abstract available.
-
High-Resolution Secretome Analysis of Chemical Hypoxia Treated Cells Identifies Putative Biomarkers of Chondrosarcoma.Proteomes. 2022 Jul 20;10(3):25. doi: 10.3390/proteomes10030025. Proteomes. 2022. PMID: 35893766 Free PMC article.
-
The neuroprotective effects of FG-4592, a hypoxia-inducible factor-prolyl hydroxylase inhibitor, against oxidative stress induced by alpha-synuclein in N2a cells.Sci Rep. 2023 Sep 20;13(1):15629. doi: 10.1038/s41598-023-42903-7. Sci Rep. 2023. PMID: 37731009 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

