Role for Kisspeptin and Neurokinin B in Regulation of Luteinizing Hormone and Testosterone Secretion in the Fetal Sheep
- PMID: 32005991
- PMCID: PMC7079722
- DOI: 10.1210/endocr/bqaa013
Role for Kisspeptin and Neurokinin B in Regulation of Luteinizing Hormone and Testosterone Secretion in the Fetal Sheep
Abstract
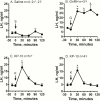
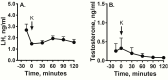
Evidence suggests that the hypothalamic-pituitary-gonadal (HPG) axis is active during the critical period for sexual differentiation of the ovine sexually dimorphic nucleus, which occurs between gestational day (GD) 60 and 90. Two possible neuropeptides that could activate the fetal HPG axis are kisspeptin and neurokinin B (NKB). We used GD85 fetal lambs to determine whether intravenous administration of kisspeptin-10 (KP-10) or senktide (NKB agonist) could elicit luteinizing hormone (LH) release. Immunohistochemistry and fluorescent in situ hybridization (FISH) were employed to localize these peptides in brains of GD60 and GD85 lamb fetuses. In anesthetized fetuses, KP-10 elicited robust release of LH that was accompanied by a delayed rise in serum testosterone in males. Pretreatment with the GnRH receptor antagonist (acyline) abolished the LH response to KP-10, confirming a hypothalamic site of action. In unanesthetized fetuses, senktide, as well as KP-10, elicited LH release. The senktide response of females was greater than that of males, indicating a difference in NKB sensitivity between sexes. Gonadotropin-releasing hormone also induced a greater LH discharge in females than in males, indicating that testosterone negative feedback is mediated through pituitary gonadotrophs. Kisspeptin and NKB immunoreactive cells in the arcuate nucleus were more abundant in females than in males. Greater than 85% of arcuate kisspeptin cells costained for NKB. FISH revealed that the majority of these were kisspeptin/NKB/dynorphin (KNDy) neurons. These results support the hypothesis that kisspeptin-GnRH signaling regulates the reproductive axis of the ovine fetus during the prenatal critical period acting to maintain a stable androgen milieu necessary for brain masculinization.
Keywords: Kisspeptin; fetal sheep; luteinizing hormone; neurokinin B; testosterone.
© Endocrine Society 2020. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures






References
-
- Foster DL, Jackson LM, Padmanabhan V. Novel concepts about normal sexual differentiation of reproductive neuroendocrine function and the developmental origins of female reproductive dysfunction: the sheep model. In: Juengel JL, Murray JF, Smith MF, eds. Reproduction in Domestic Ruminants. Vol 6. Nottingham: Nottingham University Press; 2007:83–107. - PubMed
-
- Clarke IJ, Scaramuzzi RJ, Short RV. Sexual differentiation of the brain: endocrine and behavioural responses of androgenized ewes to oestrogen. J Endocrinol. 1976;71(1):175–176. - PubMed
-
- Arnold AP, McCarthy MM. Sexual Differentiation of the Brain and Behavior: A Primer. In: Pfaff DW, Volkow ND, eds. Neuroscience in the 21st Century. New York, NY: Springer New York; 2016:1–30.
-
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19(4):323–362. - PubMed

