CD49d promotes disease progression in chronic lymphocytic leukemia: new insights from CD49d bimodal expression
- PMID: 32006000
- PMCID: PMC7228464
- DOI: 10.1182/blood.2019003179
CD49d promotes disease progression in chronic lymphocytic leukemia: new insights from CD49d bimodal expression
Abstract
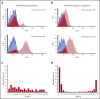
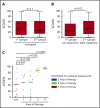
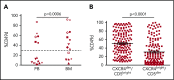
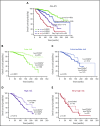
CD49d is a remarkable prognostic biomarker of chronic lymphocytic leukemia (CLL). The cutoff value for the extensively validated 30% of positive CLL cells is able to separate CLL patients into 2 subgroups with different prognoses, but it does not consider the pattern of CD49d expression. In the present study, we analyzed a cohort of 1630 CLL samples and identified the presence of ∼20% of CLL cases (n = 313) characterized by a bimodal expression of CD49d, that is, concomitant presence of a CD49d+ subpopulation and a CD49d- subpopulation. At variance with the highly stable CD49d expression observed in CLL patients with a homogeneous pattern of CD49d expression, CD49d bimodal CLL showed a higher level of variability in sequential samples, and an increase in the CD49d+ subpopulation over time after therapy. The CD49d+ subpopulation from CD49d bimodal CLL displayed higher levels of proliferation compared with the CD49d- cells; and was more highly represented in the bone marrow compared with peripheral blood (PB), and in PB CLL subsets expressing the CXCR4dim/CD5bright phenotype, known to be enriched in proliferative cells. From a clinical standpoint, CLL patients with CD49d bimodal expression, regardless of whether the CD49d+ subpopulation exceeded the 30% cutoff or not, experienced clinical behavior similar to CD49d+ CLL, both in chemoimmunotherapy (n = 1522) and in ibrutinib (n = 158) settings. Altogether, these results suggest that CD49d can drive disease progression in CLL, and that the pattern of CD49d expression should also be considered to improve the prognostic impact of this biomarker in CLL.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: D.R. received research funding from AbbVie, Janssen, and Cellestia and honoraria from AbbVie, AstraZeneca, Gilead, Janssen, Verastem, and Loxo. G.G. has acted as a consultant in advisory boards for Janssen, AbbVie, Sunesys, and AstraZeneca and in speaker’s bureaus for Janssen and AbbVie. The remaining authors declare no competing financial interests.
Figures


References
-
- Gattei V, Bulian P, Del Principe MI, et al. . Relevance of CD49d protein expression as overall survival and progressive disease prognosticator in chronic lymphocytic leukemia. Blood. 2008;111(2):865-873. - PubMed
-
- Dal Bo M, Bulian P, Bomben R, et al. . CD49d prevails over the novel recurrent mutations as independent prognosticator of overall survival in chronic lymphocytic leukemia. Leukemia. 2016;30(10):2011-2018. - PubMed
-
- Rossi D, Zucchetto A, Rossi FM, et al. . CD49d expression is an independent risk factor of progressive disease in early stage chronic lymphocytic leukemia. Haematologica. 2008;93(10):1575-1579. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

