The neuroscience of sugars in taste, gut-reward, feeding circuits, and obesity
- PMID: 32006052
- PMCID: PMC11105013
- DOI: 10.1007/s00018-020-03458-2
The neuroscience of sugars in taste, gut-reward, feeding circuits, and obesity
Abstract
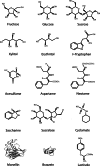
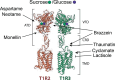
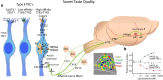
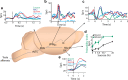
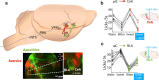
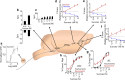
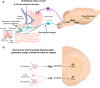
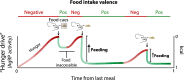
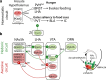
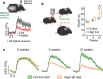
Throughout the animal kingdom sucrose is one of the most palatable and preferred tastants. From an evolutionary perspective, this is not surprising as it is a primary source of energy. However, its overconsumption can result in obesity and an associated cornucopia of maladies, including type 2 diabetes and cardiovascular disease. Here we describe three physiological levels of processing sucrose that are involved in the decision to ingest it: the tongue, gut, and brain. The first section describes the peripheral cellular and molecular mechanisms of sweet taste identification that project to higher brain centers. We argue that stimulation of the tongue with sucrose triggers the formation of three distinct pathways that convey sensory attributes about its quality, palatability, and intensity that results in a perception of sweet taste. We also discuss the coding of sucrose throughout the gustatory pathway. The second section reviews how sucrose, and other palatable foods, interact with the gut-brain axis either through the hepatoportal system and/or vagal pathways in a manner that encodes both the rewarding and of nutritional value of foods. The third section reviews the homeostatic, hedonic, and aversive brain circuits involved in the control of food intake. Finally, we discuss evidence that overconsumption of sugars (or high fat diets) blunts taste perception, the post-ingestive nutritional reward value, and the circuits that control feeding in a manner that can lead to the development of obesity.
Keywords: AgRP; Gut-reward; Hedonic taste value; LHA GABA neurons; Nutritional value; Obesity; Sugars; Sweetness.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

