Effects of the Combination of Atomoxetine and Oxybutynin on OSA Endotypic Traits
- PMID: 32006590
- PMCID: PMC7268440
- DOI: 10.1016/j.chest.2020.01.012
Effects of the Combination of Atomoxetine and Oxybutynin on OSA Endotypic Traits
Abstract
Background: We recently showed that administration of the combination of the noradrenergic drug atomoxetine plus the antimuscarinic oxybutynin (ato-oxy) prior to sleep greatly reduced OSA severity, likely by increasing upper airway dilator muscle activity during sleep. In patients with OSA who performed the ato-oxy trial with an esophageal pressure catheter to estimate ventilatory drive, the effect of the drug combination (n = 17) and of the single drugs (n = 6) was measured on the endotypic traits over a 1-night administration and compared vs placebo. This study also tested if specific traits were predictors of complete response to treatment (reduction in apnea-hypopnea index [AHI] > 50% and < 10 events/h).
Methods: The study was a double-blind, randomized, placebo-controlled trial. The arousal threshold, collapsibility (ventilation at eupneic drive [Vpassive]), ventilation at arousal threshold, and loop gain (stability of ventilatory control, LG1), were calculated during spontaneous breathing during sleep. Muscle compensation (upper airway response) was calculated as a function of ventilation at arousal threshold adjusted for Vpassive. Ventilation was expressed as a percentage of the eupneic level of ventilation (%eupnea). Data are presented as mean [95% CI].
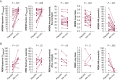
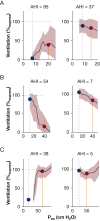
Results: Compared with placebo, ato-oxy increased Vpassive by 73 [54 to 91]%eupnea (P < .001) and muscle compensation by 29 [8 to 51]%eupnea (P = .012), reduced the arousal threshold by -9 [-14 to -3]% (P = .022) and LG1 by -11 [-22 to 2]% (P = .022). Atomoxetine alone significantly reduced arousal threshold and LG1. Both agents alone improved collapsibility (Vpassive) but not muscle compensation. Patients with lower AHI, higher Vpassive, and higher fraction of hypopneas over total events had a complete response with ato-oxy.
Findings: Ato-oxy markedly improved the measures of upper airway collapsibility, increased breathing stability, and slightly reduced the arousal threshold. Patients with relatively lower AHI and less severe upper airway collapsibility had the best chance for OSA resolution with ato-oxy.
Keywords: OSA pharmacotherapy; OSA phenotypes; noradrenergic and antimuscarinic.
Copyright © 2020 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
Could Atomoxetine-Oxybutynin, a Combination of Medications Being Explored for OSA Management, Have Any Effect on Sleep Bruxism or Jaw Muscle Tone?Chest. 2021 May;159(5):2117-2118. doi: 10.1016/j.chest.2020.11.072. Chest. 2021. PMID: 33965144 No abstract available.
-
Response.Chest. 2021 May;159(5):2118-2119. doi: 10.1016/j.chest.2020.12.042. Chest. 2021. PMID: 33965145 No abstract available.
References
-
- Chan E., Steenland H.W., Liu H. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med. 2006;174(11):1264–1273. - PubMed
-
- Grace K.P., Hughes S.W., Horner R.L. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med. 2013;187(3):311–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

