Micronized vaginal progesterone to prevent miscarriage: a critical evaluation of randomized evidence
- PMID: 32008730
- PMCID: PMC7408486
- DOI: 10.1016/j.ajog.2019.12.006
Micronized vaginal progesterone to prevent miscarriage: a critical evaluation of randomized evidence
Abstract
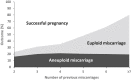
Progesterone is essential for the maintenance of pregnancy. Several small trials have suggested that progesterone supplementation may reduce the risk of miscarriage in women with recurrent or threatened miscarriage. Cochrane Reviews summarized the evidence and found that the trials were small with substantial methodologic weaknesses. Since then, the effects of first-trimester use of vaginal micronized progesterone have been evaluated in 2 large, high-quality, multicenter placebo-controlled trials, one targeting women with unexplained recurrent miscarriages (the PROMISE [PROgesterone in recurrent MIScarriagE] trial) and the other targeting women with early pregnancy bleeding (the PRISM [PRogesterone In Spontaneous Miscarriage] trial). The PROMISE trial studied 836 women from 45 hospitals in the United Kingdom and the Netherlands and found a 3% greater live birth rate with progesterone but with substantial statistical uncertainty. The PRISM trial studied 4153 women from 48 hospitals in the United Kingdom and found a 3% greater live birth rate with progesterone, but with a P value of .08. A key finding, first observed in the PROMISE trial, and then replicated in the PRISM trial, was that treatment with vaginal micronized progesterone 400 mg twice daily was associated with increasing live birth rates according to the number of previous miscarriages. Prespecified PRISM trial subgroup analysis in women with the dual risk factors of previous miscarriage(s) and current pregnancy bleeding fulfilled all 11 conditions for credible subgroup analysis. For the subgroup of women with a history of 1 or more miscarriage(s) and current pregnancy bleeding, the live birth rate was 75% (689/914) with progesterone vs 70% (619/886) with placebo (rate difference 5%; risk ratio, 1.09, 95% confidence interval, 1.03-1.15; P=.003). The benefit was greater for the subgroup of women with 3 or more previous miscarriages and current pregnancy bleeding; live birth rate was 72% (98/137) with progesterone vs 57% (85/148) with placebo (rate difference 15%; risk ratio, 1.28, 95% confidence interval, 1.08-1.51; P=.004). No short-term safety concerns were identified from the PROMISE and PRISM trials. Therefore, women with a history of miscarriage who present with bleeding in early pregnancy may benefit from the use of vaginal micronized progesterone 400 mg twice daily. Women and their care providers should use the findings for shared decision-making.
Keywords: bleeding; luteal phase deficiency; meta-analysis; recurrent miscarriage; threatened miscarriage; vaginal micronized progesterone.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures







References
-
- Di Renzo G.C., Giardina I., Cleriici G., Mattei A., Alajmi A.H., Gerli S. The role of progesterone in maternal and fetal medicine. Gynecol Endocrinol. 2012;28:925–932. - PubMed
-
- American College of Obstetricians and Gynecologists ACOG practice bulletin—clinical management guidelines for obstetricians–gynecologists: early pregnancy loss. November 2018. https://www.acog.org/-/media/Practice-Bulletins/Committee-on-Practice-Bu... Available at: Accessed Jan. 9, 2020.
-
- National Institute for Health and Care Excellence NICE guideline [NG126]. Ectopic pregnancy and miscarriage: diagnosis and initial management. 2019. https://www.nice.org.uk/guidance/ng126 Available at: Accessed Jan. 9, 2020. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

