Muscle Weakness in Myositis: MicroRNA-Mediated Dystrophin Reduction in a Myositis Mouse Model and Human Muscle Biopsies
- PMID: 32009304
- PMCID: PMC7384101
- DOI: 10.1002/art.41215
Muscle Weakness in Myositis: MicroRNA-Mediated Dystrophin Reduction in a Myositis Mouse Model and Human Muscle Biopsies
Abstract
Objective: Muscle inflammation is a feature in myositis and Duchenne muscular dystrophy (DMD). Autoimmune mechanisms are thought to contribute to muscle weakness in patients with myositis. However, a lack of correlation between the extent of inflammatory cell infiltration and muscle weakness indicates that nonimmune pathologic mechanisms may play a role. The present study focused on 2 microRNA (miRNA) sets previously identified as being elevated in the muscle of patients with DMD-an "inflammatory" miRNA set that is dampened with glucocorticoids, and a "dystrophin-targeting" miRNA set that inhibits dystrophin translation-to test the hypothesis that these miRNAs are similarly dysregulated in the muscle of patients with myositis, and could contribute to muscle weakness and disease severity.
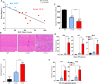
Methods: A major histocompatibility complex class I-transgenic mouse model of myositis was utilized to study gene and miRNA expression and histologic features in the muscle tissue, with the findings validated in human muscle biopsy tissue from 6 patients with myositis. Mice were classified as having mild or severe myositis based on transgene expression, body weight, histologic disease severity, and muscle strength/weakness.
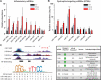
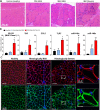
Results: In mice with severe myositis, muscle tissue showed mononuclear cell infiltration along with elevated expression of type I interferon and NF-κB-regulated genes, including Tlr7 (3.8-fold increase, P < 0.05). Furthermore, mice with severe myositis showed elevated expression of inflammatory miRNAs (miR-146a, miR-142-3p, miR-142-5p, miR-455-3p, and miR-455-5p; ~3-40-fold increase, P < 0.05) and dystrophin-targeting miRNAs (miR-146a, miR-146b, miR-31, and miR-223; ~3-38-fold increase, P < 0.05). Bioinformatics analyses of chromatin immunoprecipitation sequencing (ChIP-seq) data identified at least one NF-κB consensus element within the promoter/enhancer regions of these miRNAs. Western blotting and immunofluorescence analyses of the muscle tissue from mice with severe myositis demonstrated reduced levels of dystrophin. In addition, elevated levels of NF-κB-regulated genes, TLR7, and miRNAs along with reduced dystrophin levels were observed in muscle biopsy tissue from patients with histologically severe myositis.
Conclusion: These data demonstrate that an acquired dystrophin deficiency may occur through NF-κB-regulated miRNAs in myositis, thereby suggesting a unifying theme in which muscle injury, inflammation, and weakness are perpetuated both in myositis and in DMD.
© 2020 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures



Similar articles
-
The molecular basis of skeletal muscle weakness in a mouse model of inflammatory myopathy.Arthritis Rheum. 2012 Nov;64(11):3750-9. doi: 10.1002/art.34625. Arthritis Rheum. 2012. PMID: 22806328 Free PMC article.
-
Inhibition of myogenic microRNAs 1, 133, and 206 by inflammatory cytokines links inflammation and muscle degeneration in adult inflammatory myopathies.Arthritis Rheumatol. 2014 Apr;66(4):1022-33. doi: 10.1002/art.38292. Arthritis Rheumatol. 2014. PMID: 24757153
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
miR-146a deficiency does not aggravate muscular dystrophy in mdx mice.Skelet Muscle. 2019 Aug 14;9(1):22. doi: 10.1186/s13395-019-0207-0. Skelet Muscle. 2019. PMID: 31412923 Free PMC article.
-
Biomarker Potential of Extracellular miRNAs in Duchenne Muscular Dystrophy.Trends Mol Med. 2017 Nov;23(11):989-1001. doi: 10.1016/j.molmed.2017.09.002. Epub 2017 Oct 5. Trends Mol Med. 2017. PMID: 28988850 Review.
Cited by
-
Improved skeletal muscle fatigue resistance in experimental autoimmune myositis mice following high-intensity interval training.Arthritis Res Ther. 2022 Jun 27;24(1):156. doi: 10.1186/s13075-022-02846-2. Arthritis Res Ther. 2022. PMID: 35761371 Free PMC article.
-
Decreased dystrophin expression and elevated dystrophin-targeting miRNAs in anti-HMGCR immune-mediated necrotizing myopathy.Acta Neuropathol. 2023 Oct;146(4):655-658. doi: 10.1007/s00401-023-02619-4. Epub 2023 Aug 5. Acta Neuropathol. 2023. PMID: 37543531 No abstract available.
-
Vamorolone improves Becker muscular dystrophy and increases dystrophin protein in bmx model mice.iScience. 2023 Jun 16;26(7):107161. doi: 10.1016/j.isci.2023.107161. eCollection 2023 Jul 21. iScience. 2023. PMID: 37534133 Free PMC article.
-
Idiopathic inflammatory myopathy and non-coding RNA.Front Immunol. 2023 Sep 6;14:1227945. doi: 10.3389/fimmu.2023.1227945. eCollection 2023. Front Immunol. 2023. PMID: 37744337 Free PMC article. Review.
-
The glucocorticoid receptor acts locally to protect dystrophic muscle and heart during disease.Dis Model Mech. 2024 May 1;17(5):dmm050397. doi: 10.1242/dmm.050397. Epub 2024 May 21. Dis Model Mech. 2024. PMID: 38770680 Free PMC article.
References
-
- Meyer A, Meyer N, Schaeffer M, Gottenberg JE, Geny B, Sibilia J. Incidence and prevalence of inflammatory myopathies: a systematic review. Rheumatology (Oxford) 2015;54:50–63. - PubMed
-
- Nagaraju K, Casciola‐Rosen L, Rosen A, Thompson C, Loeffler L, Parker T, et al. The inhibition of apoptosis in myositis and in normal muscle cells. J Immunol 2000;164:5459–65. - PubMed
-
- Dalakas MC. Inflammatory muscle diseases. N Engl J Med 2015;373:393–4. - PubMed
-
- Karpati G, Pouliot Y, Carpenter S. Expression of immunoreactive major histocompatibility complex products in human skeletal muscles. Ann Neurol 1988;23:64–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

