Fimbria-Fornix Volume Is Associated With Spatial Memory and Olfactory Identification in Humans
- PMID: 32009912
- PMCID: PMC6971190
- DOI: 10.3389/fnsys.2019.00087
Fimbria-Fornix Volume Is Associated With Spatial Memory and Olfactory Identification in Humans
Abstract
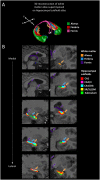
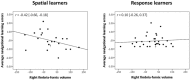
White matter pathways that surround the hippocampus comprise its afferent and efferent connections, and are therefore crucial in mediating the function of the hippocampus. We recently demonstrated a role for the hippocampus in both spatial memory and olfactory identification in humans. In the current study, we focused our attention on the fimbria-fornix white matter bundle and investigated its relationship with spatial memory and olfactory identification. We administered a virtual navigation task and an olfactory identification task to 55 young healthy adults and measured the volume of the fimbria-fornix. We found that the volume of the right fimbria-fornix and its subdivisions is correlated with both navigational learning and olfactory identification in those who use hippocampus-based spatial memory strategies, and not in those who use caudate nucleus-based navigation strategies. These results are consistent with our recent finding that spatial memory and olfaction rely on similar neural networks and structures.
Keywords: fimbria-fornix; hippocampus; navigation; olfaction; spatial memory; white matter.
Copyright © 2020 Dahmani, Courcot, Near, Patel, Amaral, Chakravarty and Bohbot.
Figures


References
-
- Amaral R. S., Park M. T. M., Devenyi G. A., Lynn V., Pipitone J., Winterburn J., et al. (2018). Manual segmentation of the fornix, fimbria, and alveus on high-resolution 3T MRI: application via fully-automated mapping of the human memory circuit white and grey matter in healthy and pathological aging. Neuroimage 170, 132–150. 10.1016/j.neuroimage.2016.10.027 - DOI - PubMed
LinkOut - more resources
Full Text Sources

