Microarray Strategies for Exploring Bacterial Surface Glycans and Their Interactions With Glycan-Binding Proteins
- PMID: 32010066
- PMCID: PMC6972965
- DOI: 10.3389/fmicb.2019.02909
Microarray Strategies for Exploring Bacterial Surface Glycans and Their Interactions With Glycan-Binding Proteins
Abstract
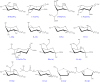
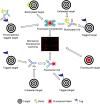
Bacterial surfaces are decorated with distinct carbohydrate structures that may substantially differ among species and strains. These structures can be recognized by a variety of glycan-binding proteins, playing an important role in the bacteria cross-talk with the host and invading bacteriophages, and also in the formation of bacterial microcolonies and biofilms. In recent years, different microarray approaches for exploring bacterial surface glycans and their recognition by proteins have been developed. A main advantage of the microarray format is the inherent miniaturization of the method, which allows sensitive and high-throughput analyses with very small amounts of sample. Antibody and lectin microarrays have been used for examining bacterial glycosignatures, enabling bacteria identification and differentiation among strains. In addition, microarrays incorporating bacterial carbohydrate structures have served to evaluate their recognition by diverse host/phage/bacterial glycan-binding proteins, such as lectins, effectors of the immune system, or bacterial and phagic cell wall lysins, and to identify antigenic determinants for vaccine development. The list of samples printed in the arrays includes polysaccharides, lipopoly/lipooligosaccharides, (lipo)teichoic acids, and peptidoglycans, as well as sequence-defined oligosaccharide fragments. Moreover, microarrays of cell wall fragments and entire bacterial cells have been developed, which also allow to study bacterial glycosylation patterns. In this review, examples of the different microarray platforms and applications are presented with a view to give the current state-of-the-art and future prospects in this field.
Keywords: antibodies; bacterial glycans; bacterial interactions; immune system; lectins; microarrays; vaccine development.
Copyright © 2020 Campanero-Rhodes, Palma, Menéndez and Solís.
Figures




References
-
- Blanco Y., Prieto-Ballesteros O., Gómez M. J., Moreno-Paz M., García-Villadangos M., Rodríguez-Manfredi J. A., et al. (2012). Prokaryotic communities and operating metabolisms in the surface and the permafrost of Deception Island (Antarctica). Environ. Microbiol. 14 2495–2510. 10.1111/j.1462-2920.2012.02767.x - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

