Antimicrobial Activity of Clinically Isolated Bacterial Species Against Staphylococcus aureus
- PMID: 32010080
- PMCID: PMC6975196
- DOI: 10.3389/fmicb.2019.02977
Antimicrobial Activity of Clinically Isolated Bacterial Species Against Staphylococcus aureus
Abstract
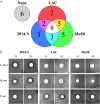
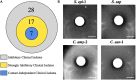
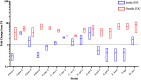
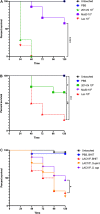
Bacteria often exist in polymicrobial communities where they compete for limited resources. Intrinsic to this competition is the ability of some species to inhibit or kill their competitors. This phenomenon is pervasive throughout the human body where commensal bacteria block the colonization of incoming microorganisms. In this regard, molecular epidemiological and microbiota-based studies suggest that species-specific interactions play a critical role in the prevention of nasal colonization of the opportunistic pathogen Staphylococcus aureus. Despite this, S. aureus exists as part of the microbiota of ∼25% of the population, suggesting that the interplay between S. aureus and commensals can be complex. Microbiota studies indicate that several bacterial genera are negatively correlated with S. aureus colonization. While these studies paint a broad overview of bacterial presence, they often fail to identify individual species-specific interactions; a greater insight in this area could aid the development of novel antimicrobials. As a proof of concept study designed to identify individual bacterial species that possess anti-S. aureus activity, we screened a small collection of clinical isolates from the Walter Reed National Military Medical Center for the ability to inhibit multiple S. aureus strains. We found that the majority of the isolates (82%) inhibited at least one S. aureus strain; 23% inhibited all S. aureus strains tested. In total, seven isolates mediated inhibitory activity that was independent of physical contact with S. aureus, and seven isolates mediated bactericidal activity. 16S rRNA based-sequencing revealed that the inhibitory isolates belonged to the Acinetobacter, Agromyces, Corynebacterium, Microbacteria, Mycobacterium, and Staphylococcus genera. Unexpectedly, these included seven distinct Acinetobacter baumannii isolates, all of which showed heterogeneous degrees of anti-S. aureus activity. Defined mechanistic studies on specific isolates revealed that the inhibitory activity was retained in conditioned cell free medium (CCFM) derived from the isolates. Furthermore, CCFM obtained from S. saprophyticus significantly decreased mortality of S. aureus-infected Galleria mellonella caterpillars. While future studies will seek to define the molecular mechanisms of the inhibitory activities, our current findings support the study of polymicrobial interactions as a strategy to understand bacterial competition and to identify novel therapeutics against S. aureus and other pathogens.
Keywords: MRSA; Staphylococcus aureus; bacterial interaction; clinical isolates; polymicrobial interactions.
Copyright © 2020 Hardy, Bansal, Hewlett, Arora, Schaffer, Kamau, Bennett and Merrell.
Figures



Similar articles
-
Corynebacterium pseudodiphtheriticum Exploits Staphylococcus aureus Virulence Components in a Novel Polymicrobial Defense Strategy.mBio. 2019 Jan 8;10(1):e02491-18. doi: 10.1128/mBio.02491-18. mBio. 2019. PMID: 30622190 Free PMC article.
-
Competition among Nasal Bacteria Suggests a Role for Siderophore-Mediated Interactions in Shaping the Human Nasal Microbiota.Appl Environ Microbiol. 2019 May 2;85(10):e02406-18. doi: 10.1128/AEM.02406-18. Print 2019 May 15. Appl Environ Microbiol. 2019. PMID: 30578265 Free PMC article.
-
Effects of intestinal colonization by Clostridium difficile and Staphylococcus aureus on microbiota diversity in healthy individuals in China.BMC Infect Dis. 2018 May 3;18(1):207. doi: 10.1186/s12879-018-3111-z. BMC Infect Dis. 2018. PMID: 29724187 Free PMC article.
-
The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota.Nat Rev Microbiol. 2017 Oct 12;15(11):675-687. doi: 10.1038/nrmicro.2017.104. Nat Rev Microbiol. 2017. PMID: 29021598 Review.
-
The role of proteinaceous toxins secreted by Staphylococcus aureus in interbacterial competition.FEMS Microbes. 2024 Feb 28;5:xtae006. doi: 10.1093/femsmc/xtae006. eCollection 2024. FEMS Microbes. 2024. PMID: 38495077 Free PMC article. Review.
Cited by
-
Temperature influences commensal-pathogen dynamics in a nasal epithelial cell co-culture model.mSphere. 2024 Jan 30;9(1):e0058923. doi: 10.1128/msphere.00589-23. Epub 2024 Jan 5. mSphere. 2024. PMID: 38179905 Free PMC article.
-
The greater wax moth Galleria mellonella: biology and use in immune studies.Pathog Dis. 2020 Nov 23;78(9):ftaa057. doi: 10.1093/femspd/ftaa057. Pathog Dis. 2020. PMID: 32970818 Free PMC article. Review.
-
Application of bacteriocins in food preservation and infectious disease treatment for humans and livestock: a review.RSC Adv. 2020 Oct 23;10(64):38937-38964. doi: 10.1039/d0ra06161a. eCollection 2020 Oct 21. RSC Adv. 2020. PMID: 35518417 Free PMC article. Review.
-
Development of Decellularized Fish Skin Scaffold Decorated with Biosynthesized Silver Nanoparticles for Accelerated Burn Wound Healing.Int J Biomater. 2023 Jan 31;2023:8541621. doi: 10.1155/2023/8541621. eCollection 2023. Int J Biomater. 2023. PMID: 36760230 Free PMC article.
-
Maternal Diet May Modulate Breast Milk Microbiota-A Case Study in a Group of Colombian Women.Microorganisms. 2023 Jul 14;11(7):1812. doi: 10.3390/microorganisms11071812. Microorganisms. 2023. PMID: 37512984 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

