Topical Therapeutic Efficacy of Ebselen Against Multidrug-Resistant Staphylococcus aureus LT-1 Targeting Thioredoxin Reductase
- PMID: 32010088
- PMCID: PMC6974526
- DOI: 10.3389/fmicb.2019.03016
Topical Therapeutic Efficacy of Ebselen Against Multidrug-Resistant Staphylococcus aureus LT-1 Targeting Thioredoxin Reductase
Abstract
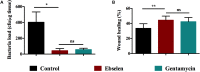
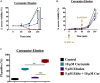
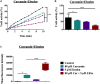
As a thiol-dependent enzyme, thioredoxin reductase (TrxR) is a promising antibacterial drug target. Ebselen, an organo-selenium with well-characterized toxicology and pharmacology, was recently reported to have potent antibacterial activity against Staphylococcus aureus. In this paper, we demonstrated that ebselen has strong bactericidal activity against multidrug-resistant (MDR) S. aureus based on taking TrxR as a major target and disruption of the redox microenvironment. Further, the topical therapeutic efficacy of ebselen for staphylococcal skin infections was assessed in a rat model. Treatment with ebselen significantly reduced the bacterial load and the expression of pro-inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1 beta (IL-1β) in S. aureus skin lesions; further, wound healing and pathological changes were obvious improved in ebselen-treated rats compare to controls. Finally, ebselen was found to sensitize S. aureus to curcumin, which may be due to their synergistic effects in inhibiting bacterial TrxR. Altogether, ebselen is an effective topical antibacterial agent in animal model of MDR S. aureus LT-1 skin infection. This may lay the foundation for further analysis and development of ebselen as an antibacterial agent for topical treatment of MDR staphylococcal infections.
Keywords: Staphylococcus aureus; curcumin; ebselen; thioredoxin reductase; topical treatment.
Copyright © 2020 Dong, Zhou, Wang, Li, Zhao, Ren, Lu, Wang, Holmgren and Zou.
Figures



Similar articles
-
Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections.Sci Rep. 2015 Jun 26;5:11596. doi: 10.1038/srep11596. Sci Rep. 2015. PMID: 26111644 Free PMC article.
-
Synergistic therapeutic efficacy of ebselen and silver ions against multidrug-resistant Acinetobacter baumannii-induced urinary tract infections.Metallomics. 2020 Jun 24;12(6):860-867. doi: 10.1039/d0mt00091d. Metallomics. 2020. PMID: 32452501
-
Depletion of multidrug-resistant uropathogenic Escherichia coli BC1 by ebselen and silver ion.J Cell Mol Med. 2020 Nov;24(22):13139-13150. doi: 10.1111/jcmm.15920. Epub 2020 Sep 25. J Cell Mol Med. 2020. PMID: 32975381 Free PMC article.
-
Selenocysteine in mammalian thioredoxin reductase and application of ebselen as a therapeutic.Free Radic Biol Med. 2018 Nov 1;127:238-247. doi: 10.1016/j.freeradbiomed.2018.05.081. Epub 2018 May 25. Free Radic Biol Med. 2018. PMID: 29807162 Review.
-
Ebselen: a thioredoxin reductase-dependent catalyst for alpha-tocopherol quinone reduction.Toxicol Appl Pharmacol. 2005 Sep 1;207(2 Suppl):103-9. doi: 10.1016/j.taap.2005.02.022. Toxicol Appl Pharmacol. 2005. PMID: 15979675 Review.
Cited by
-
One-Pot Green Synthesis and Biological Evaluation of Dimedone-Coupled 2,3-Dihydrofuran Derivatives to Divulge Their Inhibition Potential against Staphylococcal Thioredoxin Reductase Enzyme.ACS Omega. 2024 Oct 21;9(43):43414-43425. doi: 10.1021/acsomega.4c04325. eCollection 2024 Oct 29. ACS Omega. 2024. PMID: 39494007 Free PMC article.
-
Pixantrone Sensitizes Gram-Negative Pathogens to Rifampin.Microbiol Spectr. 2022 Dec 21;10(6):e0211422. doi: 10.1128/spectrum.02114-22. Epub 2022 Nov 1. Microbiol Spectr. 2022. PMID: 36318018 Free PMC article.
-
Antimicrobials Functioning through ROS-Mediated Mechanisms: Current Insights.Microorganisms. 2021 Dec 28;10(1):61. doi: 10.3390/microorganisms10010061. Microorganisms. 2021. PMID: 35056511 Free PMC article. Review.
-
Oxidative Stress-Generating Antimicrobials, a Novel Strategy to Overcome Antibacterial Resistance.Antioxidants (Basel). 2020 Apr 26;9(5):361. doi: 10.3390/antiox9050361. Antioxidants (Basel). 2020. PMID: 32357394 Free PMC article. Review.
-
The synergistic activity of SBC3 in combination with Ebselen against Escherichia coli infection.Front Pharmacol. 2022 Dec 15;13:1080281. doi: 10.3389/fphar.2022.1080281. eCollection 2022. Front Pharmacol. 2022. PMID: 36588729 Free PMC article.
References
-
- Balkhy H. H., Zowawi H., Albatshan H. A., Alshamrani M. M., Aidara-Kane A., Erlacher-Vindel E., et al. (2018). Antimicrobial resistance: a round table discussion on the “One Health” concept from the gulf cooperation council countries. part one: a focus on leadership. J. Infect. Public Health 11 771–777. 10.1016/j.jiph.2018.05.007 - DOI - PubMed
-
- Bhatia R. (2018). Antimicrobial resistance: threat, consequences and options. Natl. Med. J. India 31 133–135. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

