Prebiotic Driven Increases in IL-17A Do Not Prevent Campylobacter jejuni Colonization of Chickens
- PMID: 32010094
- PMCID: PMC6972505
- DOI: 10.3389/fmicb.2019.03030
Prebiotic Driven Increases in IL-17A Do Not Prevent Campylobacter jejuni Colonization of Chickens
Abstract
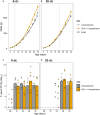
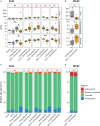
Worldwide Campylobacter jejuni is a leading cause of foodborne disease. Contamination of chicken meat with digesta from C. jejuni-positive birds during slaughter and processing is a key route of transmission to humans through the food chain. Colonization of chickens with C. jejuni elicits host innate immune responses that may be modulated by dietary additives to provide a reduction in the number of campylobacters colonizing the gastrointestinal tract and thereby reduce the likelihood of human exposure to an infectious dose. Here we report the effects of prebiotic galacto-oligosaccharide (GOS) on broiler chickens colonized with C. jejuni when challenged at either an early stage in development at 6 days of age or 20 days old when campylobacters are frequently detected in commercial flocks. GOS-fed birds had increased growth performance, but the levels of C. jejuni colonizing the cecal pouches were unchanged irrespective of the age of challenge. Dietary GOS modulated the immune response to C. jejuni by increasing cytokine IL-17A expression at colonization. Correspondingly, reduced diversity of the cecal microbiota was associated with Campylobacter colonization in GOS-fed birds. In birds challenged at 6 days-old the reduction in microbial diversity was accompanied by an increase in the relative abundance of Escherichia spp. Whilst immuno-modulation of the Th17 pro-inflammatory response did not prevent C. jejuni colonization of the intestinal tract of broiler chickens, the study highlights the potential for combinations of prebiotics, and specific competitors (synbiotics) to engage with the host innate immunity to reduce pathogen burdens.
Keywords: Campylobacter; Th17; broiler chicken; galacto-oligosaccharide; innate immunity; microbiota; prebiotic; pro-inflammatory response.
Copyright © 2020 Flaujac Lafontaine, Richards, Connerton, O’Kane, Ghaffar, Cummings, Fish and Connerton.
Figures

Similar articles
-
The effect of the timing of exposure to Campylobacter jejuni on the gut microbiome and inflammatory responses of broiler chickens.Microbiome. 2018 May 12;6(1):88. doi: 10.1186/s40168-018-0477-5. Microbiome. 2018. PMID: 29753324 Free PMC article.
-
Evaluation of a polysaccharide conjugate vaccine to reduce colonization by Campylobacter jejuni in broiler chickens.BMC Res Notes. 2015 Jun 2;8:204. doi: 10.1186/s13104-015-1203-z. BMC Res Notes. 2015. PMID: 26032784 Free PMC article.
-
Factors affecting the species of Campylobacter colonizing chickens reared for meat.J Appl Microbiol. 2020 Oct;129(4):1071-1078. doi: 10.1111/jam.14651. Epub 2020 May 21. J Appl Microbiol. 2020. PMID: 32248631
-
Re-thinking the chicken-Campylobacter jejuni interaction: a review.Avian Pathol. 2018 Aug;47(4):352-363. doi: 10.1080/03079457.2018.1475724. Epub 2018 Jun 11. Avian Pathol. 2018. PMID: 29764197 Review.
-
Poultry as a host for the zoonotic pathogen Campylobacter jejuni.Vector Borne Zoonotic Dis. 2012 Feb;12(2):89-98. doi: 10.1089/vbz.2011.0676. Epub 2011 Dec 1. Vector Borne Zoonotic Dis. 2012. PMID: 22133236 Review.
Cited by
-
Immune-Related Gene Expression Responses to In Ovo Stimulation and LPS Challenge in Two Distinct Chicken Genotypes.Genes (Basel). 2024 Dec 9;15(12):1585. doi: 10.3390/genes15121585. Genes (Basel). 2024. PMID: 39766852 Free PMC article.
-
Effects of Selected Prebiotics or Synbiotics Administered in ovo on Lymphocyte Subsets in Bursa of the Fabricius, Thymus, and Spleen in Non-Immunized and Immunized Chicken Broilers.Animals (Basel). 2021 Feb 11;11(2):476. doi: 10.3390/ani11020476. Animals (Basel). 2021. PMID: 33670391 Free PMC article.
-
Indole-3-Acetic Acid Alters Intestinal Microbiota and Alleviates Ankylosing Spondylitis in Mice.Front Immunol. 2022 Feb 4;13:762580. doi: 10.3389/fimmu.2022.762580. eCollection 2022. Front Immunol. 2022. PMID: 35185872 Free PMC article.
-
The Host Cellular Immune Response to Infection by Campylobacter Spp. and Its Role in Disease.Infect Immun. 2021 Jul 15;89(8):e0011621. doi: 10.1128/IAI.00116-21. Epub 2021 Jul 15. Infect Immun. 2021. PMID: 34031129 Free PMC article. Review.
-
The Probiotic Lactobacillus fermentum Biocenol CCM 7514 Moderates Campylobacter jejuni-Induced Body Weight Impairment by Improving Gut Morphometry and Regulating Cecal Cytokine Abundance in Broiler Chickens.Animals (Basel). 2021 Jan 19;11(1):235. doi: 10.3390/ani11010235. Animals (Basel). 2021. PMID: 33477806 Free PMC article.
References
-
- Alizadeh A., Akbari P., Difilippo E., Schols H. A., Ulfman L. H., Schoterman M. H. C., et al. (2016). The piglet as a model for studying dietary components in infant diets: effects of galacto-oligosaccharides on intestinal functions. Br. J. Nutr. 115 605–618. 10.1017/S0007114515004997 - DOI - PubMed
-
- Andreoletti O., Budka H., Buncic S., Collins J. D., Griffin J., Hald T., et al. (2010). Scientific opinion on quantification of the risk posed by broiler meat to human campylobacteriosis in the EU. EFSA J. 8:1437 10.2903/j.efsa.2010.1437 - DOI
-
- Aviagen Performance Objectives (2014). Ross 308 Broiler Performance Objectives. Scotland, UK: Aviagen Limited Newbridge, Midlothian EH28 8SZ.
-
- Awad W. A., Mann E., Dzieciol M., Hess C., Schmitz-Esser S., Wagner M., et al. (2016). Age-related differences in the luminal and mucosa-associated gut microbiome of broiler chickens and shifts associated with Campylobacter jejuni infection. Front. Cell. Infect. Microbiol. 6:154. 10.3389/fcimb.2016.00154 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

